Quantitating the Transfer of the HTLV-1 p8 Protein Between T-Cells by Flow Cytometry
- PMID: 29563906
- PMCID: PMC5850991
- DOI: 10.3389/fmicb.2018.00400
Quantitating the Transfer of the HTLV-1 p8 Protein Between T-Cells by Flow Cytometry
Abstract
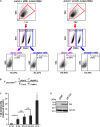
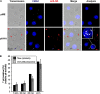
The Human T-cell leukemia virus type 1 (HTLV-1)-encoded accessory protein p8 is cleaved from the precursor protein p12 encoded by the HTLV-1 open reading frame I. Both p12 and p8 are thought to contribute to efficient viral persistence. Mechanistically, p8 induces T-cell conjugates and cellular conduits. The latter are considered to facilitate transfer of p8 to target cells and virus transmission. Transfer of p8 between p8-expressing T-cells and recipient cells has been analyzed by immunofluorescence and live imaging. However, automatic quantitation of p8-transfer between cells has not been studied yet. Here we developed a novel method allowing time saving quantitation of p8 transfer between cells by flow cytometry. After establishing a protocol for the detection of intracellular p8 by flow cytometry and validation of p8 protein expression by western blot and immunofluorescence, we set up a co-culture assay between p8-expressing donor Jurkat T-cells and recipient Jurkat T-cells that had been prestained with a well-retained live cell dye. Upon quantitating the amount of p8 positive recipient cells with regard to the percentage of p8 expressing donor cells, time course experiments confirmed that p8 is rapidly transferred between Jurkat T-cells. We found that p8 enters approximately 5% of recipient T-cells immediately upon co-culture for 5 min. Prolonged co-culture for up to 24 h revealed an increase of relative p8 transfer to approximately 23% of the recipient cells. Immunofluorescence analysis of co-culture experiments and manual quantitation of p8 expression in fluorescence images confirmed the validity of the flow cytometry based assay. Application of the new assay revealed that manipulation of actin polymerization significantly decreased p8 transfer between Jurkat T-cells suggesting an important role of actin dynamics contributing to p8 transfer. Further, transfer of p8 to co-cultured T-cells varies between different donor cell types since p8 transfer could hardly been detected in co-cultures of 293T donor cells with Jurkat acceptor cells. In summary, our novel assay allows automatic and rapid quantitation of p8 transfer to target cells and might thus contribute to a better understanding of cellular processes and dynamics regulating p8 transfer and HTLV-1 transmission.
Keywords: HTLV-1; flow cytometry; p8; protein transport; virus transmission.
Figures




Similar articles
-
Transfer of HTLV-1 p8 and Gag to target T-cells depends on VASP, a novel interaction partner of p8.PLoS Pathog. 2020 Sep 30;16(9):e1008879. doi: 10.1371/journal.ppat.1008879. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32997728 Free PMC article.
-
Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins.Mol Aspects Med. 2010 Oct;31(5):333-43. doi: 10.1016/j.mam.2010.07.001. Epub 2010 Jul 29. Mol Aspects Med. 2010. PMID: 20673780 Free PMC article. Review.
-
Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission.Proc Natl Acad Sci U S A. 2010 Nov 30;107(48):20738-43. doi: 10.1073/pnas.1009635107. Epub 2010 Nov 12. Proc Natl Acad Sci U S A. 2010. PMID: 21076035 Free PMC article.
-
The Tax-Inducible Actin-Bundling Protein Fascin Is Crucial for Release and Cell-to-Cell Transmission of Human T-Cell Leukemia Virus Type 1 (HTLV-1).PLoS Pathog. 2016 Oct 24;12(10):e1005916. doi: 10.1371/journal.ppat.1005916. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27776189 Free PMC article.
-
Molecular Mechanisms of HTLV-1 Cell-to-Cell Transmission.Viruses. 2016 Mar 9;8(3):74. doi: 10.3390/v8030074. Viruses. 2016. PMID: 27005656 Free PMC article. Review.
Cited by
-
HTLV-1 infection of myeloid cells: from transmission to immune alterations.Retrovirology. 2019 Dec 23;16(1):45. doi: 10.1186/s12977-019-0506-x. Retrovirology. 2019. PMID: 31870397 Free PMC article. Review.
-
Roles of non-coding RNAs in intercellular crosstalk in cardiovascular diseases.Korean J Physiol Pharmacol. 2023 Jul 1;27(4):289-298. doi: 10.4196/kjpp.2023.27.4.289. Korean J Physiol Pharmacol. 2023. PMID: 37386827 Free PMC article. Review.
-
Complete Rescue of HTLV-1p12KO Infectivity by Depletion of Monocytes Together with NK and CD8+ T Cells.Pathogens. 2024 Mar 29;13(4):292. doi: 10.3390/pathogens13040292. Pathogens. 2024. PMID: 38668247 Free PMC article.
-
Collagen IV (COL4A1, COL4A2), a Component of the Viral Biofilm, Is Induced by the HTLV-1 Oncoprotein Tax and Impacts Virus Transmission.Front Microbiol. 2019 Oct 23;10:2439. doi: 10.3389/fmicb.2019.02439. eCollection 2019. Front Microbiol. 2019. PMID: 31708905 Free PMC article.
-
Inhibition of Tunneling Nanotube (TNT) Formation and Human T-cell Leukemia Virus Type 1 (HTLV-1) Transmission by Cytarabine.Sci Rep. 2018 Jul 24;8(1):11118. doi: 10.1038/s41598-018-29391-w. Sci Rep. 2018. PMID: 30042514 Free PMC article.
References
-
- Chevalier S. A., Turpin J., Cachat A., Afonso P. V., Gessain A., Brady J. N., et al. (2014). Gem-induced cytoskeleton remodeling increases cellular migration of HTLV-1-infected cells, formation of infected-to-target T-cell conjugates and viral transmission. PLoS Pathog. 10:e1003917. 10.1371/journal.ppat.1003917 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

