Seroprevalence of Neutralizing Antibodies against Human Adenovirus Type-5 and Chimpanzee Adenovirus Type-68 in Cancer Patients
- PMID: 29563911
- PMCID: PMC5845880
- DOI: 10.3389/fimmu.2018.00335
Seroprevalence of Neutralizing Antibodies against Human Adenovirus Type-5 and Chimpanzee Adenovirus Type-68 in Cancer Patients
Abstract
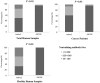
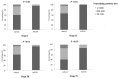
Since the preclinical results about chimpanzee adenovirus serotype-68 (AdC68)-based vaccine showed an encouraging results, it reminded us that AdC68 may be a suitable cancer vaccine vector. Previous study indicated that the seroprevalence of neutralizing antibodies (NAbs) against adenovirus was different between cancer patients and healthy volunteers. Knowledge regarding the prevalence rates of AdC68 NAbs for cancer patients is lacking. Therefore, assessing the preexistence of NAbs against AdC68 in cancer patients could provide useful insights for developing future AdC68-based cancer vaccines. In this study, 440 patients with different pathological types of tumors and 204 healthy adult volunteers were enrolled to evaluate the NAbs against AdC68 and human adenovirus serotype-5 (AdHu5). The seroprevalence of NAbs against AdC68 was much lower than that against AdHu5 in cancer subjects (43.64 vs. 67.05%, P < 0.01). The seroprevalence rates of NAbs to AdC68 in the cancer subjects were statistically higher than those detected in the healthy adult volunteers (43.64 vs. 23.53%, P = 0.000). The seroprevalence rates of AdC68 NAbs were much lower in lung, laryngeal, esophageal, and cervical cancer patients compared with oropharyngeal, colon, and rectal cancer patients. Furthermore, the seroprevalence rates of AdC68 NAbs were much lower in lung adenocarcinoma patients than in lung squamous cell carcinoma patients (35.00 vs. 70.00%, P < 0.05). No significant difference in the AdC68 NAbs among patients with different clinical stages of cancer was detected. The percentage of NAbs against AdC68 was significantly lower than that against AdHu5 (P < 0.05) in stage-I, -II, and -III cancer patients. No significant difference between the percentage of NAbs against AdC68 and AdHu5 in the subjects with stage-IV cancer was detected. The study also demonstrated the distribution of AdHu5 and AdC68 NAb titers for the positive samples. It showed that very low NAb titers against AdC68 with respect to AdHu5 in both healthy subjects and cancer subjects, especially in lung, laryngeal, esophageal, gastric, and cervical carcinomas. Also, the titer of NAbs against AdC68 was significantly lower than that against AdHu5 in the same clinical stage and age group (P < 0.05). Taken together, the present study showed that NAbs against AdC68 is much lower than AdHu5, especially in lung adenocarcinoma, laryngeal cancer, esophageal cancer, and cervical cancer patients. These results provided strong support for candidating AdC68 as a suitable vector of cancer vaccines.
Keywords: cancer immunotherapy; cancer vaccine; chimpanzee adenovirus serotype-68; human adenovirus serotype-5; neutralizing antibodies.
Figures

References
-
- Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med (2013) 5(205):205ra134.10.1126/scitranslmed.3006843 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

