Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp
- PMID: 29563968
- PMCID: PMC5851066
- DOI: 10.1186/s13068-018-1065-4
Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp
Abstract
Background: Schizochytrium sp. is a marine microalga with great potential as a promising sustainable source of lipids rich in docosahexaenoic acid (DHA). This organism's lipid accumulation machinery can be induced by various stress conditions, but this stress induction usually comes at the expense of lower biomass in industrial fermentations. Moreover, oxidative damage induced by various environmental stresses can result in the peroxidation of lipids, and especially polyunsaturated fatty acids, which causes unstable DHA production, but is often ignored in fermentation processes. Therefore, it is urgent to develop new production strains that not only have a high DHA production capacity, but also possess strong antioxidant defenses.
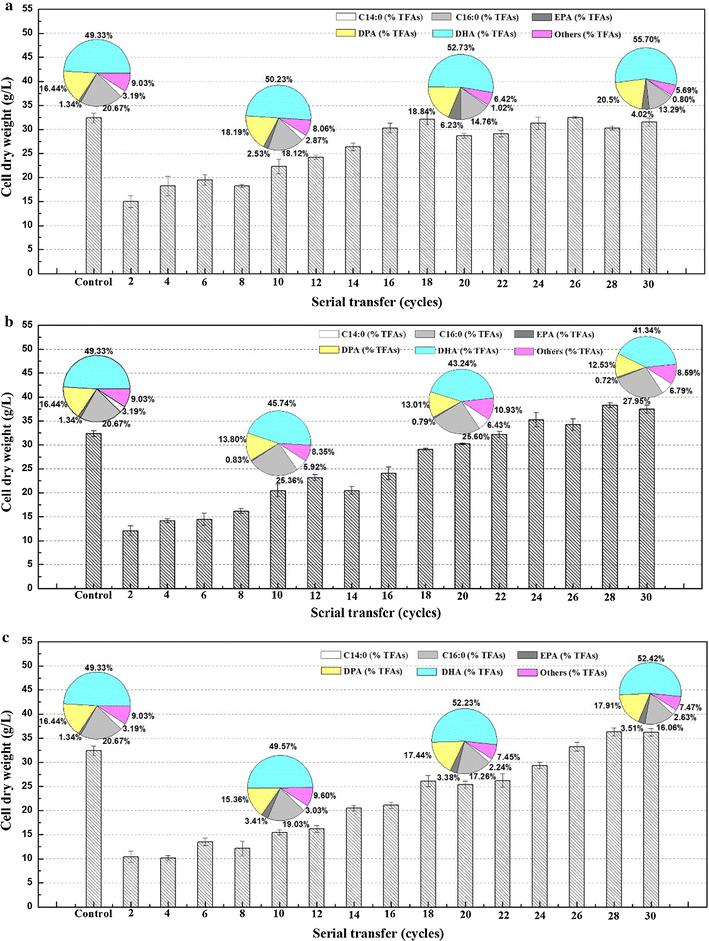
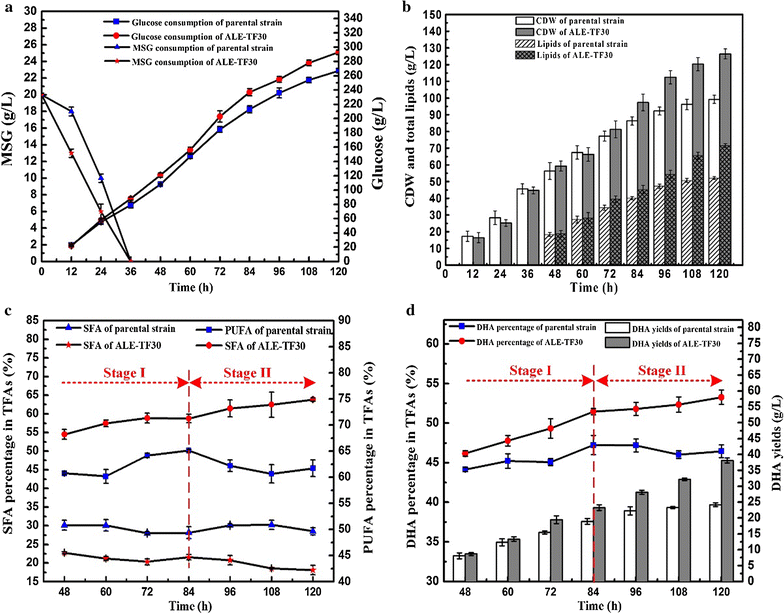
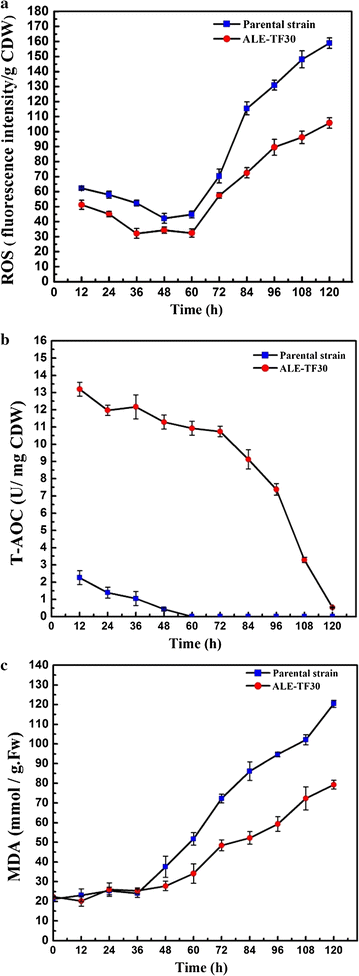
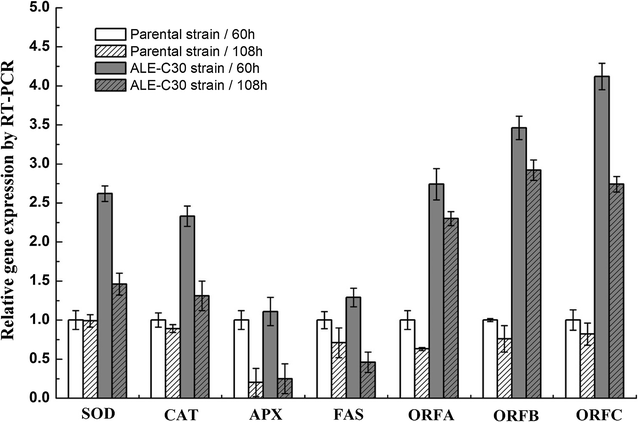
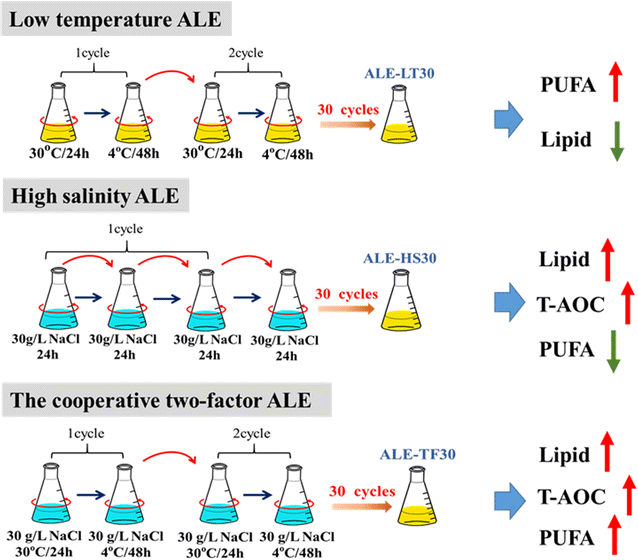
Results: Adaptive laboratory evolution (ALE) is an effective method for the development of beneficial phenotypes in industrial microorganisms. Here, a novel cooperative two-factor ALE strategy based on concomitant low temperature and high salinity was applied to improve the production capacity of Schizochytrium sp. Low-temperature conditions were used to improve the DHA content, and high salinity was applied to stimulate lipid accumulation and enhance the antioxidative defense systems of Schizochytrium sp. After 30 adaptation cycles, a maximal cell dry weight of 126.4 g/L and DHA yield of 38.12 g/L were obtained in the endpoint strain ALE-TF30, which was 27.42 and 57.52% higher than parental strain, respectively. Moreover, the fact that ALE-TF30 had the lowest concentrations of reactive oxygen species and malondialdehyde among all strains indicated that lipid peroxidation was greatly suppressed by the evolutionary process. Accordingly, the ALE-TF30 strain exhibited an overall increase of gene expression levels of antioxidant enzymes and polyketide synthases compared to the parental strain.
Conclusion: This study provides important clues on how to overcome the negative effects of lipid peroxidation on DHA production in Schizochytrium sp. Taken together, the cooperative two-factor ALE process can not only increase the accumulation of lipids rich in DHA, but also prevent the loss of produced lipid caused by lipid peroxidation. The strategy proposed here may provide a new and alternative direction for the industrial cultivation of oil-producing microalgae.
Keywords: Adaptive evolution; Antioxidant enzyme; Docosahexaenoic acid; Lipid peroxidation; Schizochytrium sp..
Figures

Similar articles
-
Adaptive evolution of Schizochytrium sp. by continuous high oxygen stimulations to enhance docosahexaenoic acid synthesis.Bioresour Technol. 2016 Jul;211:374-81. doi: 10.1016/j.biortech.2016.03.093. Epub 2016 Mar 22. Bioresour Technol. 2016. PMID: 27030957
-
Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis.Bioresour Technol. 2018 Nov;267:438-444. doi: 10.1016/j.biortech.2018.07.079. Epub 2018 Jul 17. Bioresour Technol. 2018. PMID: 30032058
-
Zinc Finger Protein LipR Represses Docosahexaenoic Acid and Lipid Biosynthesis in Schizochytrium sp.Appl Environ Microbiol. 2022 Mar 22;88(6):e0206321. doi: 10.1128/aem.02063-21. Epub 2022 Feb 2. Appl Environ Microbiol. 2022. PMID: 35108079 Free PMC article.
-
Metabolic regulation strategies for enhancing microbial docosahexaenoic acid production by Schizochytrium sp.World J Microbiol Biotechnol. 2025 Apr 28;41(5):142. doi: 10.1007/s11274-025-04268-z. World J Microbiol Biotechnol. 2025. PMID: 40289231 Review.
-
The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp.: from biochemical to genetic respects.Appl Microbiol Biotechnol. 2020 Nov;104(22):9433-9447. doi: 10.1007/s00253-020-10927-y. Epub 2020 Sep 26. Appl Microbiol Biotechnol. 2020. PMID: 32978687 Review.
Cited by
-
Fatty Acids Derivatives From Eukaryotic Microalgae, Pathways and Potential Applications.Front Microbiol. 2021 Sep 29;12:718933. doi: 10.3389/fmicb.2021.718933. eCollection 2021. Front Microbiol. 2021. PMID: 34659147 Free PMC article. Review.
-
Key Enzymes in Fatty Acid Synthesis Pathway for Bioactive Lipids Biosynthesis.Front Nutr. 2022 Feb 23;9:851402. doi: 10.3389/fnut.2022.851402. eCollection 2022. Front Nutr. 2022. PMID: 35284441 Free PMC article. Review.
-
Comparative Transcriptomic Analysis on the Effect of Sesamol on the Two-Stages Fermentation of Aurantiochytrium sp. for Enhancing DHA Accumulation.Mar Drugs. 2024 Aug 16;22(8):371. doi: 10.3390/md22080371. Mar Drugs. 2024. PMID: 39195487 Free PMC article.
-
Adaptive Laboratory Evolution of Microalgae: A Review of the Regulation of Growth, Stress Resistance, Metabolic Processes, and Biodegradation of Pollutants.Front Microbiol. 2021 Aug 18;12:737248. doi: 10.3389/fmicb.2021.737248. eCollection 2021. Front Microbiol. 2021. PMID: 34484172 Free PMC article. Review.
-
Characterization and optimization of endogenous lipid accumulation in Chlorella vulgaris SDEC-3M ability to rapidly accumulate lipid for reversing nightly lipid loss.Biotechnol Biofuels. 2019 Jun 18;12:151. doi: 10.1186/s13068-019-1493-9. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31236138 Free PMC article.
References
-
- Tacon AGJ, Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture. 2008;285:146–158. doi: 10.1016/j.aquaculture.2008.08.015. - DOI
-
- Patil KP, Gogate PR. Improved synthesis of docosahexaenoic acid (dha) using schizochytrium limacinum sr21 and sustainable media. Chem Eng J. 2015;268:187–196. doi: 10.1016/j.cej.2015.01.050. - DOI
-
- Lari Z, Moradi-Kheibari N, Ahmadzadeh H, Abrishamchi P, Moheimani NR, Murry MA. Bioprocess engineering of microalgae to optimize lipid production through nutrient management. J Appl Phycol. 2016;28:3235–3250. doi: 10.1007/s10811-016-0884-6. - DOI
-
- Duan X. Salt-induced osmotic stress for lipid overproduction in batch culture of chlorella vulgaris. Afr J Biotechnol. 2014;11:7072–7078.
LinkOut - more resources
Full Text Sources
Other Literature Sources

