Recent progress in the racemic and enantioselective synthesis of monofluoroalkene-based dipeptide isosteres
- PMID: 29564002
- PMCID: PMC5753175
- DOI: 10.3762/bjoc.13.262
Recent progress in the racemic and enantioselective synthesis of monofluoroalkene-based dipeptide isosteres
Abstract
Monofluoroalkenes are fluorinated motifs that can be used to replace amide bonds. In order to be incorporated into peptides, it is normally necessary to first synthesize a dipeptide where the amide bond has been replaced with a monofluoroalkene. In that context, this review will present the racemic and enantioselective synthesis of monofluoroalkene-based dipeptide isosteres described since 2007. Some applications of those compounds will also be presented.
Keywords: dipeptide isosteres; monofluoroalkene-based amide bonds; monofluoroalkenes; peptides; synthesis.
Figures





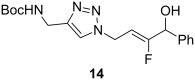











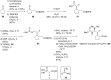
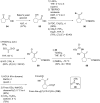



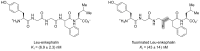
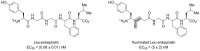
References
-
- Taguchi T, Yanai H. Fluorinated Moieties for Replacement of Amide and Peptide Bonds. In: Ojima I, editor. Fluorine in Medicinal Chemistry and Chemical Biology. Blackwell Publishing Inc.; 2009. pp. 257–290. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
