A Brønsted base-promoted diastereoselective dimerization of azlactones
- PMID: 29564003
- PMCID: PMC5753058
- DOI: 10.3762/bjoc.13.264
A Brønsted base-promoted diastereoselective dimerization of azlactones
Abstract
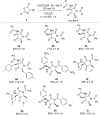
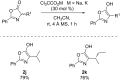
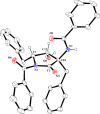
A novel Brønsted base system for the diastereoselective dimerization of azlactones using trichloroacetate salts and acetonitrile has been developed. Desired products were obtained in good yields (60-93%) and with up to >19:1 dr after one hour of reaction. Additionally, the relative stereochemistry of the major dimer was assigned as being trans, by X-ray crystallographic analysis. The kinetic reaction profile was determined by using 1H NMR reaction monitoring and revealed a second order overall kinetic profile. Furthermore, by employing this methodology, a diastereoselective total synthesis of a functionalized analogue of streptopyrrolidine was accomplished in 65% overall yield.
Keywords: azlactones; diasteoreselective synthesis; dimerization; kinetics; streptopyrrolidine analogue.
Figures


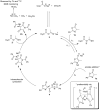

 vs time for the dimerization of azlactone 1a.
vs time for the dimerization of azlactone 1a.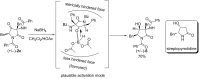
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
