Reagent-controlled regiodivergent intermolecular cyclization of 2-aminobenzothiazoles with β-ketoesters and β-ketoamides
- PMID: 29564009
- PMCID: PMC5753174
- DOI: 10.3762/bjoc.13.270
Reagent-controlled regiodivergent intermolecular cyclization of 2-aminobenzothiazoles with β-ketoesters and β-ketoamides
Abstract
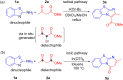
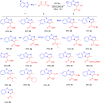
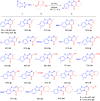
Two regiodivergent approaches to intermolecular cyclization of 2-aminobenzothiazoles with β-ketoesters and amides have been developed, controlled by the reagents employed. With the Brønsted base KOt-Bu and CBrCl3 as radical initiator, benzo[d]imidazo[2,1-b]thiazoles are synthesized via attack at the α-carbon and keto carbon of the β-ketoester moiety. In contrast, switching to the Lewis acid catalyst, In(OTf)3, results in the regioselective nucleophilic attack at both carbonyl groups forming benzo[4,5]thiazolo[3,2-a]pyrimidin-4-ones instead.
Keywords: cyclization; fused-ring systems; indium; radical; regiodivergent.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
