Position-dependent impact of hexafluoroleucine and trifluoroisoleucine on protease digestion
- PMID: 29564015
- PMCID: PMC5753150
- DOI: 10.3762/bjoc.13.279
Position-dependent impact of hexafluoroleucine and trifluoroisoleucine on protease digestion
Abstract
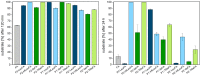
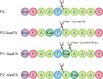
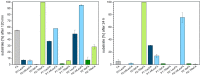
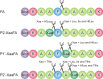
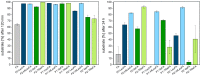
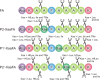
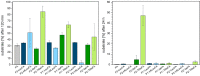
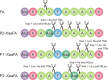
Rapid digestion by proteases limits the application of peptides as therapeutics. One strategy to increase the proteolytic stability of peptides is the modification with fluorinated amino acids. This study presents a systematic investigation of the effects of fluorinated leucine and isoleucine derivatives on the proteolytic stability of a peptide that was designed to comprise substrate specificities of different proteases. Therefore, leucine, isoleucine, and their side-chain fluorinated variants were site-specifically incorporated at different positions of this peptide resulting in a library of 13 distinct peptides. The stability of these peptides towards proteolysis by α-chymotrypsin, pepsin, proteinase K, and elastase was studied, and this process was followed by an FL-RP-HPLC assay in combination with mass spectrometry. In a few cases, we observed an exceptional increase in proteolytic stability upon introduction of the fluorine substituents. The opposite phenomenon was observed in other cases, and this may be explained by specific interactions of fluorinated residues with the respective enzyme binding sites. Noteworthy is that 5,5,5-trifluoroisoleucine is able to significantly protect peptides from proteolysis by all enzymes included in this study when positioned N-terminal to the cleavage site. These results provide valuable information for the application of fluorinated amino acids in the design of proteolytically stable peptide-based pharmaceuticals.
Keywords: fluorinated amino acids; hexafluoroleucine; peptide drugs; protease stability; trifluoroisoleucine.
Figures

References
-
- Uhlig T, Kyprianou T, Martinelli F G, Oppici C A, Heiligers D, Hills D, Calvo X R, Verhaert P. EuPa Open Proteomics. 2014;4:58–69. doi: 10.1016/j.euprot.2014.05.003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
