Reducing rates of Clostridium difficile infection by switching to a stand-alone NAAT with clear sampling criteria
- PMID: 29564088
- PMCID: PMC5848539
- DOI: 10.1186/s13756-018-0332-2
Reducing rates of Clostridium difficile infection by switching to a stand-alone NAAT with clear sampling criteria
Abstract
Background: Clostridium difficile infection is an important cause of morbidity and mortality but the optimal method of diagnosis for both patient management and infection prevention remains controversial.
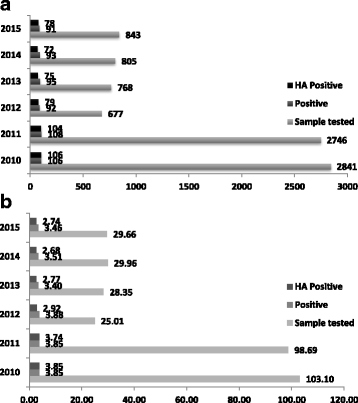
Methods: Our hospital made a decision to switch from the use of toxin immunoassay to a stand-alone nucleic acid test. This change was accompanied by the provision of clear sampling guidance and rejection criteria and this study aimed to assess the impact of that change. We analysed sample numbers, numbers of positive results, and the proportion of cases assessed as healthcare acquired over a 6-year period during which the testing method was changed from a toxin A/B immunoassay to a stand-alone commercial nucleic acid test after the first two years.
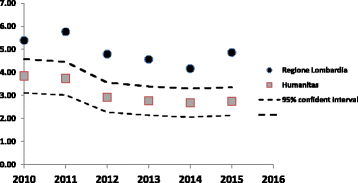
Results: Sample numbers and numbers of cases assessed as healthcare acquired fell following the introduction of the nucleic acid test and sampling guidance, while infection rates in other hospitals in the same region remained relatively stable.
Conclusions: It is our opinion that the use of a highly sensitive assay together with clear sampling guidance offers the optimal approach to patient management and best use of isolation facilities.
Keywords: Clostridium difficile; Hospital CDI; NAAT test; Prevention.
Conflict of interest statement
Not applicable in this study as analysis was done on data requested for clinical purpose.For the present retrospective analysis formal consent is not required.Not applicableThe authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Can a toxin gene NAAT be used to predict toxin EIA and the severity of Clostridium difficile infection?Antimicrob Resist Infect Control. 2017 Dec 19;6:127. doi: 10.1186/s13756-017-0283-z. eCollection 2017. Antimicrob Resist Infect Control. 2017. PMID: 29270290 Free PMC article.
-
Preventable Patient Harm: a Multidisciplinary, Bundled Approach to Reducing Clostridium difficile Infections While Using a Glutamate Dehydrogenase/Toxin Immunochromatographic Assay/Nucleic Acid Amplification Test Diagnostic Algorithm.J Clin Microbiol. 2018 Aug 27;56(9):e00625-18. doi: 10.1128/JCM.00625-18. Print 2018 Sep. J Clin Microbiol. 2018. PMID: 29997201 Free PMC article.
-
Nucleic Acid Amplification Test Quantitation as Predictor of Toxin Presence in Clostridium difficile Infection.J Clin Microbiol. 2018 Feb 22;56(3):e01316-17. doi: 10.1128/JCM.01316-17. Print 2018 Mar. J Clin Microbiol. 2018. PMID: 29237788 Free PMC article.
-
Optimizing the Laboratory Diagnosis of Clostridium difficile Infection.Clin Lab Med. 2015 Jun;35(2):299-312. doi: 10.1016/j.cll.2015.02.003. Epub 2015 Mar 18. Clin Lab Med. 2015. PMID: 26004644 Review.
-
Rapid detection of Clostridium difficile toxins and laboratory diagnosis of Clostridium difficile infections.Infection. 2017 Jun;45(3):255-262. doi: 10.1007/s15010-016-0940-9. Epub 2016 Sep 6. Infection. 2017. PMID: 27601055 Review.
Cited by
-
Clostridium difficile colonization among patients with clinically significant diarrhea and no identifiable cause of diarrhea.Infect Control Hosp Epidemiol. 2018 Nov;39(11):1330-1333. doi: 10.1017/ice.2018.225. Epub 2018 Sep 18. Infect Control Hosp Epidemiol. 2018. PMID: 30226126 Free PMC article.
References
-
- Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–18. - PubMed
-
- Mermel LA, Jefferson J, Blanchard K, Parenteau S, Mathis B, Chapin K, Machan JT. ReducingClostridium difficile incidence, colectomies, and mortality in the hospital setting: a successful multidisciplinary approach. Jt Comm J Qual Patient Saf. 2013;39(7):298–305. doi: 10.1016/S1553-7250(13)39042-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

