Planar Cell Polarity Signaling in Mammalian Cardiac Morphogenesis
- PMID: 29564519
- PMCID: PMC5959767
- DOI: 10.1007/s00246-018-1860-5
Planar Cell Polarity Signaling in Mammalian Cardiac Morphogenesis
Abstract
The mammalian heart is the first organ to form and is critical for embryonic survival and development. With an occurrence of 1%, congenital heart defects (CHDs) are also the most common birth defects in humans, and major cause of childhood morbidity and mortality (Hoffman and Kaplan in J Am Coll Cardiol 39(12):1890-1900, 2002; Samanek in Cardiol Young 10(3):179-185, 2000). Understanding how the heart forms will not only help to determine the etiology and to design diagnostic and therapeutic approaches for CHDs, but may also provide insight into regenerative medicine to repair injured adult hearts. Mammalian heart development requires precise orchestration of growth, differentiation, and morphogenesis to remodel a simple linear heart tube into an intricate, four-chambered heart with properly connected pulmonary artery and aorta, a structural basis for establishing the pulmonary and systemic circulation. Here we will review the recent advance in our understanding of how the planar cell polarity pathway, a highly conserved morphogenetic engine in vertebrates, regulates polarized morphogenetic processes to contribute to both the arterial and venous poles development of the heart.
Conflict of interest statement
Conflict of Interest: None.
Figures
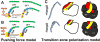
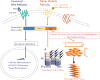
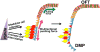
Similar articles
-
Getting to the heart of planar cell polarity signaling.Birth Defects Res A Clin Mol Teratol. 2011 Jun;91(6):460-7. doi: 10.1002/bdra.20792. Epub 2011 Apr 28. Birth Defects Res A Clin Mol Teratol. 2011. PMID: 21538810 Review.
-
A molecular and genetic outline of cardiac morphogenesis.Acta Physiol (Oxf). 2013 Apr;207(4):588-615. doi: 10.1111/apha.12061. Epub 2013 Feb 1. Acta Physiol (Oxf). 2013. PMID: 23297764 Review.
-
Tissue-tissue interactions during morphogenesis of the outflow tract.Pediatr Cardiol. 2010 Apr;31(3):408-13. doi: 10.1007/s00246-009-9611-2. Epub 2009 Dec 29. Pediatr Cardiol. 2010. PMID: 20039033 Free PMC article. Review.
-
Signaling pathways controlling second heart field development.Circ Res. 2009 Apr 24;104(8):933-42. doi: 10.1161/CIRCRESAHA.109.194464. Circ Res. 2009. PMID: 19390062 Review.
-
Planar cell polarity signaling pathway in congenital heart diseases.J Biomed Biotechnol. 2011;2011:589414. doi: 10.1155/2011/589414. Epub 2011 Oct 29. J Biomed Biotechnol. 2011. PMID: 22131815 Free PMC article. Review.
Cited by
-
Mesodermal fibronectin controls cell shape, polarity, and mechanotransduction in the second heart field during cardiac outflow tract development.Dev Cell. 2025 Jan 6;60(1):62-84.e7. doi: 10.1016/j.devcel.2024.09.017. Epub 2024 Oct 15. Dev Cell. 2025. PMID: 39413783
-
Is Transposition of the Great Arteries Associated With Shortening of the Intrapericardial Portions of the Great Arterial Trunks? An Echocardiographic Analysis on Newborn Infants With Simple Transposition of the Great Arteries to Explore an Animal Model-Based Hypothesis on Human Beings.J Am Heart Assoc. 2021 Aug 3;10(15):e019334. doi: 10.1161/JAHA.120.019334. Epub 2021 Jul 19. J Am Heart Assoc. 2021. PMID: 34278802 Free PMC article.
-
Disrupted endosomal trafficking of the Vangl-Celsr polarity complex underlies congenital anomalies in Xenopus trachea-esophageal morphogenesis.Dev Cell. 2025 May 21:S1534-5807(25)00286-2. doi: 10.1016/j.devcel.2025.04.026. Online ahead of print. Dev Cell. 2025. PMID: 40412385
-
The Effect of N6-Methyladenosine Regulators and m6A Reader YTHDC1-Mediated N6-Methyladenosine Modification Is Involved in Oxidative Stress in Human Aortic Dissection.Oxid Med Cell Longev. 2023 Feb 9;2023:3918393. doi: 10.1155/2023/3918393. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36819785 Free PMC article.
-
Planar cell polarity signaling regulates polarized second heart field morphogenesis to promote both arterial and venous pole septation.Development. 2019 Oct 9;146(20):dev181719. doi: 10.1242/dev.181719. Development. 2019. PMID: 31488563 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

