Functional and Morphological Analysis of OFF Bipolar Cells
- PMID: 29564792
- PMCID: PMC6291506
- DOI: 10.1007/978-1-4939-7720-8_15
Functional and Morphological Analysis of OFF Bipolar Cells
Abstract
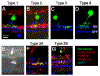
Retinal first-order neurons, photoreceptors, receive visual inputs and convert them to neural signals. The second-order neurons, bipolar cells then sort out the visual signals and encode them through multiple neural streams. Approximately 15 morphological types of bipolar cells have been identified, which are thought to encode different aspects of visual signals such as motion and color (Ichinose et al. J Neurosci 34(26):8761-8771, 2014; Euler et al. Nat Rev Neurosci 15(8):507-519, 2014). To investigate functional aspects of OFF bipolar cells, single cell recordings are preferred; however, bipolar cells in the mouse retina are small and hard to distinguish from other types of cells. Here, we describe our methodology and tips for immunohistochemistry and patch clamp recordings for analyzing light-evoked responses in each type of OFF bipolar cell.
Keywords: Immunohistochemistry; Light responses; Neurobiotin; OFF bipolar cells; Patch clamp; Retinal slice preparation.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

