Overexpression of a SBP-Box Gene (VpSBP16) from Chinese Wild Vitis Species in Arabidopsis Improves Salinity and Drought Stress Tolerance
- PMID: 29565279
- PMCID: PMC5979544
- DOI: 10.3390/ijms19040940
Overexpression of a SBP-Box Gene (VpSBP16) from Chinese Wild Vitis Species in Arabidopsis Improves Salinity and Drought Stress Tolerance
Abstract
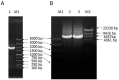
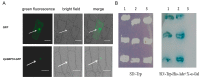
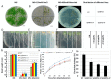
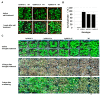
Salinity and drought are two major abiotic stresses that limit grape productivity. Responses to stress in grape are known to be regulated by several families of transcription factors. However, little is known about the role of grape Squamosa promoter binding protein (SBP)-box transcription factor genes in response to abiotic stress. To better understand the functions of the grape SBP-box genes in abiotic stress tolerance, a full-length complementary DNA (cDNA) sequence of the putative SBP-box transcription factor gene, VpSBP16 was amplified from Chinese wild grapevine Vitis pseudoreticulata clone "Baihe-35-1". We observed that the VpSBP16 protein fused to the green fluorescent protein (GFP) reporter accumulated in the nucleus when transiently expressed in onion epidermal cells. Moreover, VpSBP16 was shown to have transcriptional activation activity using a yeast trans-activation assay. We performed a VpSBP16 functional analysis through the characterization of transgenic Arabidopsis thaliana plants constitutively over-expressing VpSBP16. The transgenic lines had longer roots and the seeds had a higher germination rate than the wild type (WT) under osmotic stress. In addition, the accumulation of reactive oxygen species (ROS) of transgenic seedlings was significantly lower than WT in the transgenic lines, as was electrolyte leakage. VpSBP16 overexpression also elevated expression levels of stress-response genes involved in the salt overly sensitive (SOS) pathway. These results indicate that overexpression VpSBP16 in A. thaliana enhances tolerance of salt and drought stress during seed germination, as well in seedlings and mature plants, by regulating SOS and ROS signaling cascades.
Keywords: ROS; SOS; Vitis pseudoreticulata; VpSBP16; drought; salt.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis.Planta. 2016 Jul;244(1):59-73. doi: 10.1007/s00425-016-2489-3. Epub 2016 Mar 5. Planta. 2016. PMID: 26945856
-
The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production.Plant Cell Physiol. 2014 Dec;55(12):2060-76. doi: 10.1093/pcp/pcu133. Epub 2014 Sep 26. Plant Cell Physiol. 2014. PMID: 25261532
-
VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance.J Plant Physiol. 2015 Aug 1;185:1-12. doi: 10.1016/j.jplph.2015.05.020. Epub 2015 Jul 14. J Plant Physiol. 2015. PMID: 26264965
-
Ion homeostasis for salinity tolerance in plants: a molecular approach.Physiol Plant. 2021 Apr;171(4):578-594. doi: 10.1111/ppl.13185. Epub 2020 Aug 31. Physiol Plant. 2021. PMID: 32770745 Review.
-
Revisiting plant salt tolerance: novel components of the SOS pathway.Trends Plant Sci. 2023 Sep;28(9):1060-1069. doi: 10.1016/j.tplants.2023.04.003. Epub 2023 Apr 27. Trends Plant Sci. 2023. PMID: 37117077 Review.
Cited by
-
Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba.Genes (Basel). 2025 Apr 17;16(4):460. doi: 10.3390/genes16040460. Genes (Basel). 2025. PMID: 40282420 Free PMC article.
-
Roles of the SPL gene family and miR156 in the salt stress responses of tamarisk (Tamarix chinensis).BMC Plant Biol. 2019 Aug 22;19(1):370. doi: 10.1186/s12870-019-1977-6. BMC Plant Biol. 2019. PMID: 31438851 Free PMC article.
-
Pokkali: A Naturally Evolved Salt-Tolerant Rice Shows a Distinguished Set of lncRNAs Possibly Contributing to the Tolerant Phenotype.Int J Mol Sci. 2023 Jul 20;24(14):11677. doi: 10.3390/ijms241411677. Int J Mol Sci. 2023. PMID: 37511436 Free PMC article.
-
Identification of Pepper CaSBP08 Gene in Defense Response Against Phytophthora capsici Infection.Front Plant Sci. 2020 Feb 26;11:183. doi: 10.3389/fpls.2020.00183. eCollection 2020. Front Plant Sci. 2020. PMID: 32174944 Free PMC article.
-
Gene-Wide Analysis of Aquaporin Gene Family in Malus domestica and Heterologous Expression of the Gene MpPIP2;1 Confers Drought and Salinity Tolerance in Arabidposis thaliana.Int J Mol Sci. 2019 Jul 29;20(15):3710. doi: 10.3390/ijms20153710. Int J Mol Sci. 2019. PMID: 31362376 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

