Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must?
- PMID: 29565285
- PMCID: PMC5923436
- DOI: 10.3390/v10040142
Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must?
Abstract
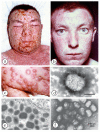
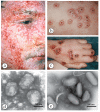
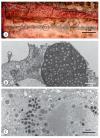
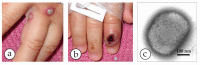
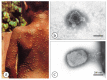
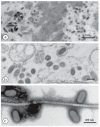
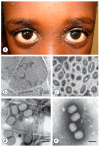
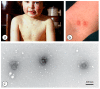
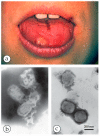
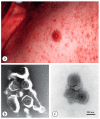
Diagnostic electron microscopy (DEM) was an essential component of viral diagnosis until the development of highly sensitive nucleic acid amplification techniques (NAT). The simple negative staining technique of DEM was applied widely to smallpox diagnosis until the world-wide eradication of the human-specific pathogen in 1980. Since then, the threat of smallpox re-emerging through laboratory escape, molecular manipulation, synthetic biology or bioterrorism has not totally disappeared and would be a major problem in an unvaccinated population. Other animal poxviruses may also emerge as human pathogens. With its rapid results (only a few minutes after arrival of the specimen), no requirement for specific reagents and its "open view", DEM remains an important component of virus diagnosis, particularly because it can easily and reliably distinguish smallpox virus or any other member of the orthopoxvirus (OPV) genus from parapoxviruses (PPV) and the far more common and less serious herpesviruses (herpes simplex and varicella zoster). Preparation, enrichment, examination, internal standards and suitable organisations are discussed to make clear its continuing value as a diagnostic technique.
Keywords: VZV); diagnostic electron microscopy (DEM); febrile vesicular rashes; herpesviruses (HSV; negative staining; orthopoxviruses (OPV); parapoxviruses (PPV); rapid viral diagnosis; skin lesions.
Conflict of interest statement
The authors declare no conflict of interests. The conclusions drawn are those of the authors and do not necessarily represent the views of our institutions.
Figures






Similar articles
-
[Rapid differential diagnosis of Orthopoxviruses and Herpesviruses based upon multiplex real-time PCR].Infez Med. 2007 Mar;15(1):47-55. Infez Med. 2007. PMID: 17515675 Italian.
-
Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: dos and don'ts.Ultrastruct Pathol. 2003 May-Jun;27(3):133-40. doi: 10.1080/01913120309932. Ultrastruct Pathol. 2003. PMID: 12775503
-
[Biological microchip for identification of orthopoxviruses and agents causing the same clinical picture as in smallpox].Vopr Virusol. 2007 Mar-Apr;52(2):41-5. Vopr Virusol. 2007. PMID: 17500239 Russian.
-
The French Armed Forces Virology Unit: A Chronological Record of Ongoing Research on Orthopoxvirus.Viruses. 2017 Dec 23;10(1):3. doi: 10.3390/v10010003. Viruses. 2017. PMID: 29295488 Free PMC article. Review.
-
[We should be prepared to smallpox re-emergence.].Vopr Virusol. 2019;64(5):206-214. doi: 10.36233/0507-4088-2019-64-5-206-214. Vopr Virusol. 2019. PMID: 32167685 Review. Russian.
Cited by
-
Electron Microscopy Methods for Virus Diagnosis and High Resolution Analysis of Viruses.Front Microbiol. 2019 Jan 7;9:3255. doi: 10.3389/fmicb.2018.03255. eCollection 2018. Front Microbiol. 2019. PMID: 30666247 Free PMC article. Review.
-
MPXV: Update on Morphological and Morphogenesis Aspects Through Transmission and Scanning Electron Microscopies and 3D Reconstruction.J Med Virol. 2025 Jan;97(1):e70180. doi: 10.1002/jmv.70180. J Med Virol. 2025. PMID: 39825732 Free PMC article.
-
Diagnostic electron microscopy in human infectious diseases - Methods and applications.J Microsc. 2025 Sep;299(3):186-205. doi: 10.1111/jmi.13370. Epub 2024 Nov 19. J Microsc. 2025. PMID: 39560601 Free PMC article. Review.
-
Role of the Laboratory in the Diagnosis of Poxvirus Infections.Adv Exp Med Biol. 2024;1451:239-252. doi: 10.1007/978-3-031-57165-7_15. Adv Exp Med Biol. 2024. PMID: 38801582 Review.
-
Concentration of SARS-CoV-2-Infected Cell Culture Supernatants for Detection of Virus-like Particles by Scanning Electron Microscopy.Viruses. 2022 Oct 28;14(11):2388. doi: 10.3390/v14112388. Viruses. 2022. PMID: 36366486 Free PMC article.
References
-
- Fenner F., Henderson D.A., Arita I., Jezek L., Ladnyi I.D. Smallpox and Its Eradication. World Health Organization; Geneva, Switzerland: 1988.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

