Sterically Shielded, Stabilized Nitrile Imine for Rapid Bioorthogonal Protein Labeling in Live Cells
- PMID: 29565582
- PMCID: PMC5901669
- DOI: 10.1021/jacs.8b00126
Sterically Shielded, Stabilized Nitrile Imine for Rapid Bioorthogonal Protein Labeling in Live Cells
Abstract
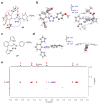
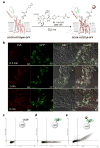
In pursuit of fast bioorthogonal reactions, reactive moieties have been increasingly employed for selective labeling of biomolecules in living systems, posing a challenge in attaining reactivity without sacrificing selectivity. To address this challenge, here we report a bioinspired strategy in which molecular shape controls the selectivity of a transient, highly reactive nitrile imine dipole. By tuning the shape of structural pendants attached to the ortho position of the N-aryl ring of diaryltetrazoles-precursors of nitrile imines, we discovered a sterically shielded nitrile imine that favors the 1,3-dipolar cycloaddition over the competing nucleophilic addition. The photogenerated nitrile imine exhibits an extraordinarily long half-life of 102 s in aqueous medium, owing to its unique molecular shape that hinders the approach of a nucleophile as shown by DFT calculations. The utility of this sterically shielded nitrile imine in rapid (∼1 min) bioorthogonal labeling of glucagon receptor in live mammalian cells was demonstrated.
Figures




Similar articles
-
Direct observation of a photoinduced nonstabilized nitrile imine structure in the solid state.J Am Chem Soc. 2009 Dec 23;131(50):18036-7. doi: 10.1021/ja9094523. J Am Chem Soc. 2009. PMID: 19928921 Free PMC article.
-
Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations.Int J Mol Sci. 2023 Jan 9;24(2):1289. doi: 10.3390/ijms24021289. Int J Mol Sci. 2023. PMID: 36674803 Free PMC article.
-
Photoisomerization-enhanced 1,3-dipolar cycloaddition of carbon-bridged octocyclic azobenzene with photo-released nitrile imine for peptide stapling and imaging in live cells.Org Biomol Chem. 2020 Aug 7;18(29):5602-5607. doi: 10.1039/d0ob01027h. Epub 2020 Jul 10. Org Biomol Chem. 2020. PMID: 32647842
-
Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells.Acc Chem Res. 2011 Sep 20;44(9):828-39. doi: 10.1021/ar200021p. Epub 2011 May 20. Acc Chem Res. 2011. PMID: 21609129 Free PMC article. Review.
-
Nitrile groups as vibrational probes of biomolecular structure and dynamics: an overview.Phys Chem Chem Phys. 2009 Oct 1;11(37):8119-32. doi: 10.1039/b908588b. Epub 2009 Jul 31. Phys Chem Chem Phys. 2009. PMID: 19756266 Review.
Cited by
-
Bioorthogonal Ligations and Cleavages in Chemical Biology.ChemistryOpen. 2020 Aug 14;9(8):835-853. doi: 10.1002/open.202000128. eCollection 2020 Aug. ChemistryOpen. 2020. PMID: 32817809 Free PMC article. Review.
-
Catalytic Activation of Bioorthogonal Chemistry with Light (CABL) Enables Rapid, Spatiotemporally Controlled Labeling and No-Wash, Subcellular 3D-Patterning in Live Cells Using Long Wavelength Light.J Am Chem Soc. 2022 Feb 2;144(4):1647-1662. doi: 10.1021/jacs.1c10390. Epub 2022 Jan 24. J Am Chem Soc. 2022. PMID: 35072462 Free PMC article.
-
Toolbox for the structure-guided evolution of ferulic acid decarboxylase (FDC).Sci Rep. 2022 Mar 1;12(1):3347. doi: 10.1038/s41598-022-07110-w. Sci Rep. 2022. PMID: 35232989 Free PMC article.
-
Rapid Identification of Functional Pyrrolysyl-tRNA Synthetases via Fluorescence-Activated Cell Sorting.Int J Mol Sci. 2018 Dec 21;20(1):29. doi: 10.3390/ijms20010029. Int J Mol Sci. 2018. PMID: 30577609 Free PMC article.
-
Sterically shielded tetrazoles for a fluorogenic photoclick reaction: tuning cycloaddition rate and product fluorescence.Org Biomol Chem. 2018 Jul 25;16(29):5241-5244. doi: 10.1039/c8ob01404c. Org Biomol Chem. 2018. PMID: 29995029 Free PMC article.
References
-
- Boutureira O, Bernardes GJ. Chem Rev. 2015;115:2174–2195. - PubMed
-
- Lang K, Chin JW. Chem Rev. 2014;114:4764–4806. - PubMed
-
- Meldal M, Tornoe CW. Chem Rev. 2008;108:2952–3015. - PubMed
-
- MacKenzie DA, Sherratt AR, Chigrinova M, Cheung LL, Pezacki JP. Curr Opin Chem Biol. 2014;21:81–88. - PubMed
-
- Oliveira BL, Guo Z, Bernardes GJL. Chem Soc Rev. 2017;46:4895–4950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

