IL-27 Expression and Responsiveness in Human Uterine Epithelial Cells and Fibroblasts In Vitro and the Role of Estradiol
- PMID: 29565744
- PMCID: PMC5867512
- DOI: 10.1089/jir.2017.0038
IL-27 Expression and Responsiveness in Human Uterine Epithelial Cells and Fibroblasts In Vitro and the Role of Estradiol
Abstract
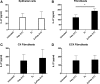
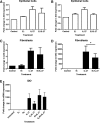
Interleukin (IL)-27 is a pleiotropic cytokine that regulates multiple aspects of innate and adaptive immunity, but whose role in immune protection of the female reproductive tract is unknown. Although not constitutively expressed by human uterine epithelial cells and fibroblasts in culture, IL-27 secretion was upregulated after treatment with the viral ligand poly (I:C) in a type I interferon (IFN)-dependent manner, with higher levels measured in fibroblasts than epithelial cells. Estradiol increased poly (I:C)-induced IL-27 production by fibroblasts, but not epithelial cells. While both cell types expressed the IL-27 receptor, only fibroblasts responded to recombinant IL-27 with increased expression of the antiviral genes, APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) and MxA, and the tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO). Estradiol inhibited IL-27-mediated induction of IDO in fibroblasts through estrogen receptor alpha, but had no effect on APOBEC3G. IL-27 pretreatment also potentiated poly (I:C) upregulation of the antiviral genes, OAS2 and APOBEC3G, in fibroblasts. Thus, IL-27 is part of the antiviral response by uterine cells against potential pathogens. The effect of estradiol on IL-27 production and sensitivity by fibroblasts demonstrates a selective hormone action on individual cell types in the uterus and suggests that IL-27 may have differential effects during the menstrual cycle.
Keywords: IL-27; epithelial cells; estradiol; poly (I:C); stromal fibroblasts; uterus.
Conflict of interest statement
No competing financial interests exist.
Figures




Similar articles
-
Estradiol-regulated innate antiviral responses of human endometrial stromal fibroblasts.Am J Reprod Immunol. 2018 Nov;80(5):e13042. doi: 10.1111/aji.13042. Epub 2018 Sep 17. Am J Reprod Immunol. 2018. PMID: 30295964 Free PMC article.
-
Sex Hormones and Aging Modulate Interferon Lambda 1 Production and Signaling by Human Uterine Epithelial Cells and Fibroblasts.Front Immunol. 2021 Sep 24;12:718380. doi: 10.3389/fimmu.2021.718380. eCollection 2021. Front Immunol. 2021. PMID: 34630393 Free PMC article.
-
Uterine epithelial cells specifically induce interferon-stimulated genes in response to polyinosinic-polycytidylic acid independently of estradiol.PLoS One. 2012;7(4):e35654. doi: 10.1371/journal.pone.0035654. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558189 Free PMC article.
-
IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells.J Immunol. 2005 Nov 15;175(10):6509-16. doi: 10.4049/jimmunol.175.10.6509. J Immunol. 2005. PMID: 16272305
-
Modulation of hepatocyte growth factor secretion in human female reproductive tract stromal fibroblasts by poly (I:C) and estradiol.Am J Reprod Immunol. 2012 Jan;67(1):44-53. doi: 10.1111/j.1600-0897.2011.01063.x. Epub 2011 Aug 23. Am J Reprod Immunol. 2012. PMID: 21883619 Free PMC article.
Cited by
-
Estradiol-regulated innate antiviral responses of human endometrial stromal fibroblasts.Am J Reprod Immunol. 2018 Nov;80(5):e13042. doi: 10.1111/aji.13042. Epub 2018 Sep 17. Am J Reprod Immunol. 2018. PMID: 30295964 Free PMC article.
-
IL-30† (IL-27A): a familiar stranger in immunity, inflammation, and cancer.Exp Mol Med. 2021 May;53(5):823-834. doi: 10.1038/s12276-021-00630-x. Epub 2021 May 28. Exp Mol Med. 2021. PMID: 34045653 Free PMC article. Review.
-
The impact of aging on innate and adaptive immunity in the human female genital tract.Aging Cell. 2021 May;20(5):e13361. doi: 10.1111/acel.13361. Epub 2021 May 5. Aging Cell. 2021. PMID: 33951269 Free PMC article. Review.
-
T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer.Oncoimmunology. 2021 Jul 8;10(1):1940675. doi: 10.1080/2162402X.2021.1940675. eCollection 2021. Oncoimmunology. 2021. PMID: 34290905 Free PMC article.
-
Epithelial Cells and Fibroblasts from the Human Female Reproductive Tract Accumulate and Release TFV and TAF to Sustain Inhibition of HIV Infection of CD4+ T cells.Sci Rep. 2019 Feb 12;9(1):1864. doi: 10.1038/s41598-018-38205-y. Sci Rep. 2019. PMID: 30755713 Free PMC article.
References
-
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8(12):1380–1389 - PubMed
-
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7(9):929–936 - PubMed
-
- Björnström L, Sjöberg M. 2005. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19(4):833–842 - PubMed
-
- Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, Hornung RL, Wang Y, Huang da W, Hu X, Lempicki RA, Imamichi T. 2013. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PLoS One 8(3):e59194. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

