Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials
- PMID: 29565804
- PMCID: PMC6023083
- DOI: 10.3390/antibiotics7020029
Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials
Erratum in
-
Correction: São-José, C. Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials. Antibiotics 2018, 7, 29.Antibiotics (Basel). 2018 Jul 4;7(3):56. doi: 10.3390/antibiotics7030056. Antibiotics (Basel). 2018. PMID: 29973495 Free PMC article.
Abstract
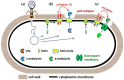
Lytic enzymes encoded by bacteriophages have been intensively explored as alternative agents for combating bacterial pathogens in different contexts. The antibacterial character of these enzymes (enzybiotics) results from their degrading activity towards peptidoglycan, an essential component of the bacterial cell wall. In fact, phage lytic products have the capacity to kill target bacteria when added exogenously in the form of recombinant proteins. However, there is also growing recognition that the natural bactericidal activity of these agents can, and sometimes needs to be, substantially improved through manipulation of their functional domains or by equipping them with new functions. In addition, often, native lytic proteins exhibit features that restrict their applicability as effective antibacterials, such as poor solubility or reduced stability. Here, I present an overview of the engineering approaches that can be followed not only to overcome these and other restrictions, but also to generate completely new antibacterial agents with significantly enhanced characteristics. As conventional antibiotics are running short, the remarkable progress in this field opens up the possibility of tailoring efficient enzybiotics to tackle the most menacing bacterial infections.
Keywords: antibacterial; antibiotic resistance; antimicrobial; antimicrobial resistance; bacteriophage; endolysin; lysin; lytic enzyme; peptidoglycan hydrolase.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy.Viruses. 2018 May 29;10(6):292. doi: 10.3390/v10060292. Viruses. 2018. PMID: 29844287 Free PMC article. Review.
-
Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications.Recent Pat Biotechnol. 2007;1(2):113-22. doi: 10.2174/187220807780809463. Recent Pat Biotechnol. 2007. PMID: 19075835 Review.
-
Treating Bacterial Infections with Bacteriophage-Based Enzybiotics: In Vitro, In Vivo and Clinical Application.Antibiotics (Basel). 2021 Dec 6;10(12):1497. doi: 10.3390/antibiotics10121497. Antibiotics (Basel). 2021. PMID: 34943709 Free PMC article. Review.
-
Phage lytic enzymes: a history.Virol Sin. 2015 Feb;30(1):26-32. doi: 10.1007/s12250-014-3549-0. Epub 2015 Feb 5. Virol Sin. 2015. PMID: 25662888 Free PMC article. Review.
-
Enzybiotics: Enzyme-Based Antibacterials as Therapeutics.Adv Exp Med Biol. 2019;1148:233-253. doi: 10.1007/978-981-13-7709-9_11. Adv Exp Med Biol. 2019. PMID: 31482502 Review.
Cited by
-
Validation and Stabilization of a Prophage Lysin of Clostridium perfringens by Using Yeast Surface Display and Coevolutionary Models.Appl Environ Microbiol. 2019 May 2;85(10):e00054-19. doi: 10.1128/AEM.00054-19. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30850429 Free PMC article.
-
The Influence of Dimerization on the Pharmacokinetics and Activity of an Antibacterial Enzyme Lysostaphin.Molecules. 2019 May 16;24(10):1879. doi: 10.3390/molecules24101879. Molecules. 2019. PMID: 31100806 Free PMC article.
-
Phage Lysins for Fighting Bacterial Respiratory Infections: A New Generation of Antimicrobials.Front Immunol. 2018 Oct 16;9:2252. doi: 10.3389/fimmu.2018.02252. eCollection 2018. Front Immunol. 2018. PMID: 30459750 Free PMC article. Review.
-
Antibacterial Activity of a Lytic Enzyme Encoded by Pseudomonas aeruginosa Double Stranded RNA Bacteriophage phiYY.Front Microbiol. 2018 Aug 3;9:1778. doi: 10.3389/fmicb.2018.01778. eCollection 2018. Front Microbiol. 2018. PMID: 30127777 Free PMC article.
-
Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy.Viruses. 2018 May 29;10(6):292. doi: 10.3390/v10060292. Viruses. 2018. PMID: 29844287 Free PMC article. Review.
References
-
- WHO The World Is Running Out of Antibiotics, WHO Report Confirms. [(accessed on 3 January 2018)];2017 Available online: http://www.who.int/mediacentre/news/releases/2017/running-out-antibiotic...
-
- O’Neill J. Tackling Drug-Restistant Infections Globally: Final Report and Recommendations. [(accessed on 1 March 2018)];2016 Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20c....
-
- Adeyi O.O., Baris E., Jonas O.B., Irwin A., Berthe F.C.J., Le Gall F.G., Marquez P.V., Nikolic I.A., Plante C.A., Schneidman M., et al. Drug-Resistant Infections: A Threat to Our Economic Future. World Bank Group; Washington, DC, USA: 2017. Final Report.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

