Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications
- PMID: 29565814
- PMCID: PMC5924518
- DOI: 10.3390/genes9040176
Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications
Abstract
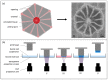
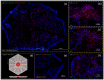
Many tissue models have been developed to mimic liver-specific functions for metabolic and toxin conversion in in vitro assays. Most models represent a 2D environment rather than a complex 3D structure similar to native tissue. To overcome this issue, spheroid cultures have become the gold standard in tissue engineering. Unfortunately, spheroids are limited in size due to diffusion barriers in their dense structures, limiting nutrient and oxygen supply. Recent developments in bioprinting techniques have enabled us to engineer complex 3D structures with perfusion-enabled channel systems to ensure nutritional supply within larger, densely-populated tissue models. In this study, we present a proof-of-concept for the feasibility of bioprinting a liver organoid by combining HepaRG and human stellate cells in a stereolithographic printing approach, and show basic characterization under static cultivation conditions. Using standard tissue engineering analytics, such as immunohistology and qPCR, we found higher albumin and cytochrome P450 3A4 (CYP3A4) expression in bioprinted liver tissues compared to monolayer controls over a two-week cultivation period. In addition, the expression of tight junctions, liver-specific bile transporter multidrug resistance-associated protein 2 (MRP2), and overall metabolism (glucose, lactate, lactate dehydrogenase (LDH)) were found to be stable. Furthermore, we provide evidence for the perfusability of the organoids' intrinsic channel system. These results motivate new approaches and further development in liver tissue engineering for advanced organ-on-a-chip applications and pharmaceutical developments.
Keywords: 3D cell-culture; bioink; bioprinting; drug development; in vitro testing; liver equivalent; stereolithography; tissue engineering; toxin testing.
Conflict of interest statement
The authors declare no conflicts of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




Similar articles
-
A multi-organ-chip co-culture of liver and testis equivalents: a first step toward a systemic male reprotoxicity model.Hum Reprod. 2020 May 1;35(5):1029-1044. doi: 10.1093/humrep/deaa057. Hum Reprod. 2020. PMID: 32390056
-
A Bioprinted Liver-on-a-Chip for Drug Screening Applications.Trends Biotechnol. 2016 Sep;34(9):681-682. doi: 10.1016/j.tibtech.2016.05.014. Epub 2016 Jun 10. Trends Biotechnol. 2016. PMID: 27291461
-
3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications.Biomacromolecules. 2015 May 11;16(5):1489-96. doi: 10.1021/acs.biomac.5b00188. Epub 2015 Apr 7. Biomacromolecules. 2015. PMID: 25806996
-
Tissue Engineering Applications of Three-Dimensional Bioprinting.Cell Biochem Biophys. 2015 Jul;72(3):777-82. doi: 10.1007/s12013-015-0531-x. Cell Biochem Biophys. 2015. PMID: 25663505 Review.
-
3D Bioprinting of Tissue/Organ Models.Angew Chem Int Ed Engl. 2016 Apr 4;55(15):4650-65. doi: 10.1002/anie.201505062. Epub 2016 Feb 19. Angew Chem Int Ed Engl. 2016. PMID: 26895542 Review.
Cited by
-
Perfusion in Organ-on-Chip Models and Its Applicability to the Replication of Spermatogenesis In Vitro.Int J Mol Sci. 2022 May 12;23(10):5402. doi: 10.3390/ijms23105402. Int J Mol Sci. 2022. PMID: 35628214 Free PMC article. Review.
-
Revolutionizing digestive system tumor organoids research: Exploring the potential of tumor organoids.J Tissue Eng. 2024 May 27;15:20417314241255470. doi: 10.1177/20417314241255470. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38808253 Free PMC article. Review.
-
Emerging Technologies in Multi-Material Bioprinting.Adv Mater. 2021 Dec;33(49):e2104730. doi: 10.1002/adma.202104730. Epub 2021 Oct 1. Adv Mater. 2021. PMID: 34596923 Free PMC article. Review.
-
Organ-on-a-Chip systems for new drugs development.ADMET DMPK. 2021 Mar 22;9(2):111-141. doi: 10.5599/admet.942. eCollection 2021. ADMET DMPK. 2021. PMID: 35299767 Free PMC article. Review.
-
Microphysiological Models for Mechanistic-Based Prediction of Idiosyncratic DILI.Cells. 2023 May 25;12(11):1476. doi: 10.3390/cells12111476. Cells. 2023. PMID: 37296597 Free PMC article. Review.
References
-
- Maschmeyer I., Lorenz A.K., Schimek K., Hasenberg T., Ramme A.P., Hübner J., Lindner M., Drewell C., Bauer S., Thomas A., et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–2699. doi: 10.1039/C5LC00392J. - DOI - PubMed
-
- Bauer S., Wennberg Huldt C., Kanebratt K.P., Durieux I., Gunne D., Andersson S., Ewart L., Haynes W.G., Maschmeyer I., Winter A., et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017;7:14620. doi: 10.1038/s41598-017-14815-w. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

