Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma
- PMID: 29565815
- PMCID: PMC5923345
- DOI: 10.3390/cancers10040090
Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma
Abstract
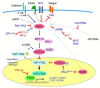
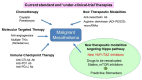
Malignant mesothelioma (MM) constitutes a very aggressive tumor that arises from the pleural or peritoneal cavities and is highly refractory to conventional therapies. Several key genetic alterations are associated with the development and progression of MM including mutations of the CDKN2A/ARF, NF2, and BAP1 tumor-suppressor genes. Notably, activating oncogene mutations are very rare; thus, it is difficult to develop effective inhibitors to treat MM. The NF2 gene encodes merlin, a protein that regulates multiple cell-signaling cascades including the Hippo pathway. MMs also exhibit inactivation of Hippo pathway components including LATS1/2, strongly suggesting that merlin-Hippo pathway dysregulation plays a key role in the development and progression of MM. Furthermore, Hippo pathway inactivation has been shown to result in constitutive activation of the YAP1/TAZ transcriptional coactivators, thereby conferring malignant phenotypes to mesothelial cells. Critical YAP1/TAZ target genes, including prooncogenic CCDN1 and CTGF, have also been shown to enhance the malignant phenotypes of MM cells. Together, these data indicate the Hippo pathway as a therapeutic target for the treatment of MM, and support the development of new strategies to effectively target the activation status of YAP1/TAZ as a promising therapeutic modality for this formidable disease.
Keywords: Hippo pathway; NF2; YAP1/TAZ; malignant mesothelioma.
Conflict of interest statement
The author received a collaboration grant from Kyowa Hakko Kirin Co., Ltd. (Tokyo, JAPAN), and Eisai Co., Ltd. (Tokyo, JAPAN). The founding sponsors had no role in the writing of the manuscript.
Figures

Similar articles
-
TAZ activation by Hippo pathway dysregulation induces cytokine gene expression and promotes mesothelial cell transformation.Oncogene. 2019 Mar;38(11):1966-1978. doi: 10.1038/s41388-018-0417-7. Epub 2018 Nov 6. Oncogene. 2019. PMID: 30401981
-
The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma.Am J Cancer Res. 2020 Dec 1;10(12):4399-4415. eCollection 2020. Am J Cancer Res. 2020. PMID: 33415007 Free PMC article.
-
Aberrant expression of NPPB through YAP1 and TAZ activation in mesothelioma with Hippo pathway gene alterations.Cancer Med. 2023 Jun;12(12):13586-13598. doi: 10.1002/cam4.6056. Epub 2023 May 11. Cancer Med. 2023. PMID: 37165917 Free PMC article.
-
Molecular pathogenesis of malignant mesothelioma.Carcinogenesis. 2013 Jul;34(7):1413-9. doi: 10.1093/carcin/bgt166. Epub 2013 May 14. Carcinogenesis. 2013. PMID: 23677068 Review.
-
NF2 alteration in mesothelioma.Front Toxicol. 2023 Apr 25;5:1161995. doi: 10.3389/ftox.2023.1161995. eCollection 2023. Front Toxicol. 2023. PMID: 37180489 Free PMC article. Review.
Cited by
-
Research progress of plant-derived natural products in thyroid carcinoma.Front Chem. 2024 Jan 10;11:1279384. doi: 10.3389/fchem.2023.1279384. eCollection 2023. Front Chem. 2024. PMID: 38268761 Free PMC article. Review.
-
LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma.Cell Death Dis. 2018 Sep 20;9(10):947. doi: 10.1038/s41419-018-0975-1. Cell Death Dis. 2018. PMID: 30237435 Free PMC article.
-
The oncogenic role of NF1 in gallbladder cancer through regulation of YAP1 stability by direct interaction with YAP1.J Transl Med. 2023 May 5;21(1):306. doi: 10.1186/s12967-023-04157-9. J Transl Med. 2023. PMID: 37147639 Free PMC article.
-
LncRNA LINC00857 regulates the progression and glycolysis in ovarian cancer by modulating the Hippo signaling pathway.Cancer Med. 2020 Nov;9(21):8122-8132. doi: 10.1002/cam4.3322. Epub 2020 Sep 12. Cancer Med. 2020. PMID: 32918541 Free PMC article.
-
Oncofetal reprogramming in tumor development and progression: novel insights into cancer therapy.MedComm (2020). 2023 Dec 2;4(6):e427. doi: 10.1002/mco2.427. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38045829 Free PMC article. Review.
References
-
- Napolitano A., Antoine D.J., Pellegrini L., Baumann F., Pagano I., Pastorino S., Goparaju C.M., Prokrym K., Canino C., Pass H.I., et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin. Cancer Res. 2016;22:3087–3096. doi: 10.1158/1078-0432.CCR-15-1130. - DOI - PMC - PubMed
-
- Husain A.N., Colby T.V., Ordonez N.G., Krausz T., Borczuk A., Cagle P.T., Chirieac L.R., Churg A., Galateau-Salle F., Gibbs A.R., et al. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2009;133:1317–1331. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

