Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions
- PMID: 29565819
- PMCID: PMC5946176
- DOI: 10.3390/nu10040391
Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions
Abstract
Proteins are macro-molecules crucial for cell life, which are made up of amino acids (AAs). In healthy people, protein synthesis and degradation are well balanced. However, in the presence of hypercatabolic stimulation (i.e., inflammation), protein breakdown increases as the resulting AAs are consumed for metabolic proposes. Indeed, AAs are biochemical totipotent molecules which, when deaminated, can be transformed into energy, lipids, carbohydrates, and/or biochemical intermediates of fundamental cycles, such as the Krebs' cycle. The biochemical consequence of hyper-catabolism is protein disarrangement, clinically evident with signs such as sarcopenia, hypalbuminemia, anaemia, infection, and altered fluid compartmentation, etc. Hypercatabolic protein disarrangement (HPD) is often underestimated by clinicians, despite correlating with increased mortality, hospitalization, and morbidity quite independent of the primary disease. Simple, cheap, repeatable measurements can be used to identify HPD. Therefore, identification and treatment of proteins' metabolic impairment with appropriate measurements and therapy is a clinical strategy that could improve the prognosis of patients with acute/chronic hypercatabolic inflammatory disease. Here, we describe the metabolism of protein and AAs in hypercatabolic syndrome, illustrating the clinical impact of protein disarrangement. We also illustrate simple, cheap, repeatable, and worldwide available measurements to identify these conditions. Finally, we provide scientific evidence for HPD nutritional treatment.
Keywords: amino acids; catabolism; inflammation; muscle wasting; protein metabolism; sarcopenia.
Conflict of interest statement
F.S.D. is the inventor and owner of US patent no. US6218420 B1: Compositions based on amino-acids for preventing and treating alimentary overload under conditions of high body nitrogen requirements, without causing calcium loss; no. US7973077 B2: Amino acid-based compositions for the treatment of pathological conditions distinguished by insufficient mitochondrial function and other patents pending on different amino acid based formulations. R.C. and E.M. are partners of the SPRINTT consortium, which is partly funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA). E.M. served as a consultant for Huron Consulting Group, Genactis, and Novartis. R.C. served as a consultant from Novartis and Nutricia. All other authors have no competing financial interests to declare.
Figures


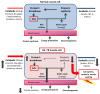

References
-
- Lehninger A.L. Principles of Biochemistry. Worth Publishers Inc.; New York, NY, USA: 1982.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

