Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis
- PMID: 29565833
- PMCID: PMC5924519
- DOI: 10.3390/genes9040177
Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis
Abstract
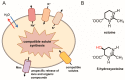
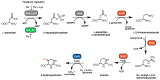
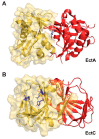
Fluctuations in environmental osmolarity are ubiquitous stress factors in many natural habitats of microorganisms, as they inevitably trigger osmotically instigated fluxes of water across the semi-permeable cytoplasmic membrane. Under hyperosmotic conditions, many microorganisms fend off the detrimental effects of water efflux and the ensuing dehydration of the cytoplasm and drop in turgor through the accumulation of a restricted class of organic osmolytes, the compatible solutes. Ectoine and its derivative 5-hydroxyectoine are prominent members of these compounds and are synthesized widely by members of the Bacteria and a few Archaea and Eukarya in response to high salinity/osmolarity and/or growth temperature extremes. Ectoines have excellent function-preserving properties, attributes that have led to their description as chemical chaperones and fostered the development of an industrial-scale biotechnological production process for their exploitation in biotechnology, skin care, and medicine. We review, here, the current knowledge on the biochemistry of the ectoine/hydroxyectoine biosynthetic enzymes and the available crystal structures of some of them, explore the genetics of the underlying biosynthetic genes and their transcriptional regulation, and present an extensive phylogenomic analysis of the ectoine/hydroxyectoine biosynthetic genes. In addition, we address the biochemistry, phylogenomics, and genetic regulation for the alternative use of ectoines as nutrients.
Keywords: biotechnology; chemical chaperones; crystal structures; enzymes; gene expression; genomics; growth temperature extremes; high salinity; osmotic stress.
Conflict of interest statement
Conflict of Interests: The authors declare no conflict of interest.
Figures





References
-
- Bremer E., Krämer R. Coping with osmotic challenges: Osmoregulation through accumulation and release of compatible solutes. In: Storz G., Hengge-Aronis R., editors. Bacterial Stress Responses. ASM Press; Washington, DC, USA: 2000. pp. 79–97.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

