Blood coagulation abnormalities in multibacillary leprosy patients
- PMID: 29565968
- PMCID: PMC5863944
- DOI: 10.1371/journal.pntd.0006214
Blood coagulation abnormalities in multibacillary leprosy patients
Abstract
Background: Leprosy is a chronic dermato-neurological disease caused by Mycobacterium leprae infection. In 2016, more than 200,000 new cases of leprosy were detected around the world, representing the most frequent cause of infectious irreversible deformities and disabilities.
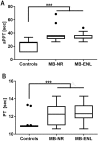
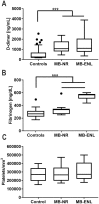
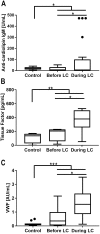
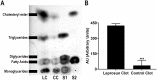
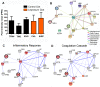
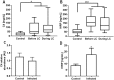
Principal findings: In the present work, we demonstrate a consistent procoagulant profile on 40 reactional and non-reactional multibacillary leprosy patients. A retrospective analysis in search of signs of coagulation abnormalities among 638 leprosy patients identified 35 leprosy patients (5.48%) which displayed a characteristic lipid-like clot formed between blood clot and serum during serum harvesting, herein named 'leprosum clot'. Most of these patients (n = 16, 45.7%) belonged to the lepromatous leprosy pole of the disease. In addition, formation of the leprosum clot was directly correlated with increased plasma levels of soluble tissue factor and von Willebrand factor. High performance thin layer chromatography demonstrated a high content of neutral lipids in the leprosum clot, and proteomic analysis demonstrated that the leprosum clot presented in these patients is highly enriched in fibrin. Remarkably, differential 2D-proteomics analysis between leprosum clots and control clots identified two proteins present only in leprosy patients clots: complement component 3 and 4 and inter-alpha-trypsin inhibitor family heavy chain-related protein (IHRP). In agreement with those observations we demonstrated that M. leprae induces hepatocytes release of IHRP in vitro.
Conclusions: We demonstrated that leprosy MB patients develop a procoagulant status due to high levels of plasmatic fibrinogen, anti-cardiolipin antibodies, von Willebrand factor and soluble tissue factor. We propose that some of these components, fibrinogen for example, presents potential as predictive biomarkers of leprosy reactions, generating tools for earlier diagnosis and treatment of these events.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

Similar articles
-
Increased chitotriosidase activity in serum of leprosy patients: association with bacillary leprosy.Clin Immunol. 2009 Jun;131(3):501-9. doi: 10.1016/j.clim.2009.02.003. Clin Immunol. 2009. PMID: 19307157
-
Differential immunoglobulin and complement levels in leprosy prior to development of reversal reaction and erythema nodosum leprosum.PLoS Negl Trop Dis. 2019 Jan 28;13(1):e0007089. doi: 10.1371/journal.pntd.0007089. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30689631 Free PMC article.
-
Increased serum circulatory levels of interleukin 17F in type 1 reactions of leprosy.J Clin Immunol. 2012 Dec;32(6):1415-20. doi: 10.1007/s10875-012-9747-3. Epub 2012 Jul 31. J Clin Immunol. 2012. PMID: 22847545
-
Erythema nodosum leprosum: reactional leprosy.Semin Cutan Med Surg. 2007 Jun;26(2):126-30. doi: 10.1016/j.sder.2007.02.010. Semin Cutan Med Surg. 2007. PMID: 17544965 Review.
-
[Histoid leprosy with erythema nodosum leprosum].Acta Leprol. 2003;12(3):107-11. Acta Leprol. 2003. PMID: 15040700 Review. French.
Cited by
-
Case Report: Glans penile necrosis in a patient with SARS-CoV-2 and leprosy infection.F1000Res. 2024 Feb 5;11:142. doi: 10.12688/f1000research.84355.4. eCollection 2022. F1000Res. 2024. PMID: 39233873 Free PMC article.
-
Diagnostic Value of Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio, and Platelet-to-Lymphocyte Ratio in the Diagnosis of Erythema Nodosum Leprosum: A Retrospective Study.Trop Med Infect Dis. 2022 Mar 2;7(3):39. doi: 10.3390/tropicalmed7030039. Trop Med Infect Dis. 2022. PMID: 35324586 Free PMC article.
-
Serum Interleukin 6 Level and Nutrition Status as Potential Predictors of Clinical Leprosy Development Among Household Contacts in Endemic Areas.Open Forum Infect Dis. 2022 Feb 2;9(3):ofac010. doi: 10.1093/ofid/ofac010. eCollection 2022 Mar. Open Forum Infect Dis. 2022. PMID: 35237701 Free PMC article.
-
Involvement of TNF-Producing CD8+ Effector Memory T Cells with Immunopathogenesis of Erythema Nodosum Leprosum in Leprosy Patients.Am J Trop Med Hyg. 2019 Feb;100(2):377-385. doi: 10.4269/ajtmh.18-0517. Am J Trop Med Hyg. 2019. PMID: 30652669 Free PMC article.
-
Host-Related Laboratory Parameters for Leprosy Reactions.Front Med (Lausanne). 2021 Oct 22;8:694376. doi: 10.3389/fmed.2021.694376. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34746168 Free PMC article. Review.
References
-
- Silva SR, Tempone AJ, Silva TP, Costa MR, Pereira GM, Lara FA, et al. Mycobacterium leprae downregulates the expression of PHEX in Schwann cells and osteoblasts. Memorias do Instituto Oswaldo Cruz. 2010;105(5):627–32. - PubMed
-
- Mattos KA, Lara FA, Oliveira VG, Rodrigues LS, D'Avila H, Melo RC, et al. Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cellular microbiology. 2011;13(2):259–73. doi: 10.1111/j.1462-5822.2010.01533.x - DOI - PubMed
-
- Mattos KA, Oliveira VC, Berredo-Pinho M, Amaral JJ, Antunes LC, Melo RC, et al. Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cellular microbiology. 2014;16(6):797–815. doi: 10.1111/cmi.12279 - DOI - PMC - PubMed
-
- Esquenazi D, Alvim IM, Pinheiro RO, Oliveira EB, Moreira Lde O, Sarno EN, et al. Correlation between Central Memory T Cell Expression and Proinflammatory Cytokine Production with Clinical Presentation of Multibacillary Leprosy Relapse. PloS one. 2015;10(5):e0127416 doi: 10.1371/journal.pone.0127416 - DOI - PMC - PubMed
-
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clinical microbiology reviews. 2006;19(2):338–81. doi: 10.1128/CMR.19.2.338-381.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

