Activation gating in HCN2 channels
- PMID: 29565972
- PMCID: PMC5863937
- DOI: 10.1371/journal.pcbi.1006045
Activation gating in HCN2 channels
Abstract
Hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels control electrical rhythmicity in specialized brain and heart cells. We quantitatively analysed voltage-dependent activation of homotetrameric HCN2 channels and its modulation by the second messenger cAMP using global fits of hidden Markovian models to complex experimental data. We show that voltage-dependent activation is essentially governed by two separable voltage-dependent steps followed by voltage-independent opening of the pore. According to this model analysis, the binding of cAMP to the channels exerts multiple effects on the voltage-dependent gating: It stabilizes the open pore, reduces the total gating charge from ~8 to ~5, makes an additional closed state outside the activation pathway accessible and strongly accelerates the ON-gating but not the OFF-gating. Furthermore, the open channel has a much slower computed OFF-gating current than the closed channel, in both the absence and presence of cAMP. Together, these results provide detailed new insight into the voltage- and cAMP-induced activation gating of HCN channels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

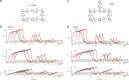


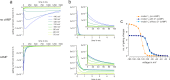

Similar articles
-
Voltage sensor movement and cAMP binding allosterically regulate an inherently voltage-independent closed-open transition in HCN channels.J Gen Physiol. 2007 Feb;129(2):175-88. doi: 10.1085/jgp.200609585. J Gen Physiol. 2007. PMID: 17261842 Free PMC article.
-
Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions.J Gen Physiol. 2001 Sep;118(3):237-50. doi: 10.1085/jgp.118.3.237. J Gen Physiol. 2001. PMID: 11524455 Free PMC article.
-
Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide.J Gen Physiol. 2001 May;117(5):491-504. doi: 10.1085/jgp.117.5.491. J Gen Physiol. 2001. PMID: 11331358 Free PMC article.
-
Regulation of HCN Ion Channels by Non-canonical Cyclic Nucleotides.Handb Exp Pharmacol. 2017;238:123-133. doi: 10.1007/164_2016_5006. Handb Exp Pharmacol. 2017. PMID: 28181007 Review.
-
The structure of the apo cAMP-binding domain of HCN4 - a stepping stone toward understanding the cAMP-dependent modulation of the hyperpolarization-activated cyclic-nucleotide-gated ion channels.FEBS J. 2018 Jun;285(12):2182-2192. doi: 10.1111/febs.14408. Epub 2018 Mar 14. FEBS J. 2018. PMID: 29444387 Review.
Cited by
-
HCN channel-mediated neuromodulation can control action potential velocity and fidelity in central axons.Elife. 2019 Sep 9;8:e42766. doi: 10.7554/eLife.42766. Elife. 2019. PMID: 31496517 Free PMC article.
-
The HCN domain couples voltage gating and cAMP response in hyperpolarization-activated cyclic nucleotide-gated channels.Elife. 2019 Nov 26;8:e49672. doi: 10.7554/eLife.49672. Elife. 2019. PMID: 31769408 Free PMC article.
-
Unravelling the intricate cooperativity of subunit gating in P2X2 ion channels.Sci Rep. 2020 Dec 10;10(1):21751. doi: 10.1038/s41598-020-78672-w. Sci Rep. 2020. PMID: 33303878 Free PMC article.
-
The HCN domain is required for HCN channel cell-surface expression and couples voltage- and cAMP-dependent gating mechanisms.J Biol Chem. 2020 Jun 12;295(24):8164-8173. doi: 10.1074/jbc.RA120.013281. Epub 2020 Apr 27. J Biol Chem. 2020. PMID: 32341127 Free PMC article.
-
Helix breaking transition in the S4 of HCN channel is critical for hyperpolarization-dependent gating.Elife. 2019 Nov 27;8:e53400. doi: 10.7554/eLife.53400. Elife. 2019. PMID: 31774399 Free PMC article.
References
-
- Gauss R., Seifert R., and Kaupp U. B.. 1998. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 393:583–587. doi: 10.1038/31248 - DOI - PubMed
-
- Ludwig A., Zong X., Jeglitsch M., Hofmann F., and Biel M.. 1998. A family of hyperpolarization-activated mammalian cation channels. Nature 393:587–591. doi: 10.1038/31255 - DOI - PubMed
-
- Santoro B., Liu D. T., Yao H., Bartsch D., Kandel E. R., Siegelbaum S. A., and Tibbs G. R.. 1998. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93:717–729. - PubMed
-
- Moosmang S., Stieber J., Zong X., Biel M., Hofmann F., and Ludwig A.. 2001. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. European journal of biochemistry / FEBS 268:1646–1652. - PubMed
-
- Santoro B., Chen S., Luthi A., Pavlidis P., Shumyatsky G. P., Tibbs G. R., and Siegelbaum S. A.. 2000. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience 20:5264–5275. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

