MKLN1 splicing defect in dogs with lethal acrodermatitis
- PMID: 29565995
- PMCID: PMC5863938
- DOI: 10.1371/journal.pgen.1007264
MKLN1 splicing defect in dogs with lethal acrodermatitis
Abstract
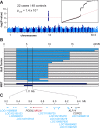
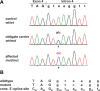
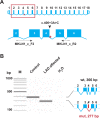
Lethal acrodermatitis (LAD) is a genodermatosis with monogenic autosomal recessive inheritance in Bull Terriers and Miniature Bull Terriers. The LAD phenotype is characterized by poor growth, immune deficiency, and skin lesions, especially at the paws. Utilizing a combination of genome wide association study and haplotype analysis, we mapped the LAD locus to a critical interval of ~1.11 Mb on chromosome 14. Whole genome sequencing of an LAD affected dog revealed a splice region variant in the MKLN1 gene that was not present in 191 control genomes (chr14:5,731,405T>G or MKLN1:c.400+3A>C). This variant showed perfect association in a larger combined Bull Terrier/Miniature Bull Terrier cohort of 46 cases and 294 controls. The variant was absent from 462 genetically diverse control dogs of 62 other dog breeds. RT-PCR analysis of skin RNA from an affected and a control dog demonstrated skipping of exon 4 in the MKLN1 transcripts of the LAD affected dog, which leads to a shift in the MKLN1 reading frame. MKLN1 encodes the widely expressed intracellular protein muskelin 1, for which diverse functions in cell adhesion, morphology, spreading, and intracellular transport processes are discussed. While the pathogenesis of LAD remains unclear, our data facilitate genetic testing of Bull Terriers and Miniature Bull Terriers to prevent the unintentional production of LAD affected dogs. This study may provide a starting point to further clarify the elusive physiological role of muskelin 1 in vivo.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: AT is affiliated with a diagnostic lab marketing genetic tests for dogs. HL owns shares of a diagnostic testing lab marketing genetic tests for dogs. CM is affiliated with a diagnostic lab marketing genetic tests for dogs. MLC is affiliated with a diagnostic lab marketing genetic tests for dogs. TL is an associate editor of PLoS Genetics.
Figures

Similar articles
-
A RAPGEF6 variant constitutes a major risk factor for laryngeal paralysis in dogs.PLoS Genet. 2019 Oct 24;15(10):e1008416. doi: 10.1371/journal.pgen.1008416. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31647804 Free PMC article.
-
Diagnostic features, confirmation and disease progression in 28 cases of lethal acrodermatitis of bull terriers.J Small Anim Pract. 2000 Nov;41(11):501-7. doi: 10.1111/j.1748-5827.2000.tb03972.x. J Small Anim Pract. 2000. PMID: 11105789
-
Analysis of the liver soluble proteome from bull terriers affected with inherited lethal acrodermatitis.Mol Genet Metab. 2007 Nov;92(3):249-57. doi: 10.1016/j.ymgme.2007.07.003. Epub 2007 Aug 10. Mol Genet Metab. 2007. PMID: 17693109 Free PMC article.
-
Serum concentrations of zinc and copper in bull terriers with lethal acrodermatitis and tail-chasing behavior.Am J Vet Res. 1997 Aug;58(8):808-10. Am J Vet Res. 1997. PMID: 9256960
-
Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides.Nucleic Acid Ther. 2014 Feb;24(1):57-68. doi: 10.1089/nat.2013.0451. Epub 2013 Dec 31. Nucleic Acid Ther. 2014. PMID: 24380394 Review.
Cited by
-
TSEN54 missense variant in Standard Schnauzers with leukodystrophy.PLoS Genet. 2019 Oct 4;15(10):e1008411. doi: 10.1371/journal.pgen.1008411. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31584937 Free PMC article.
-
Insights into the genetics of body size in the Bull Terrier.Anim Genet. 2025 Feb;56(1):e70000. doi: 10.1111/age.70000. Anim Genet. 2025. PMID: 39871672 Free PMC article.
-
GID E3 ligase supramolecular chelate assembly configures multipronged ubiquitin targeting of an oligomeric metabolic enzyme.Mol Cell. 2021 Jun 3;81(11):2445-2459.e13. doi: 10.1016/j.molcel.2021.03.025. Epub 2021 Apr 26. Mol Cell. 2021. PMID: 33905682 Free PMC article.
-
MIA3 Splice Defect in Cane Corso Dogs with Dental-Skeletal-Retinal Anomaly (DSRA).Genes (Basel). 2021 Sep 25;12(10):1497. doi: 10.3390/genes12101497. Genes (Basel). 2021. PMID: 34680893 Free PMC article.
-
Canine neuropathies: powerful spontaneous models for human hereditary sensory neuropathies.Hum Genet. 2019 May;138(5):455-466. doi: 10.1007/s00439-019-02003-x. Epub 2019 Apr 6. Hum Genet. 2019. PMID: 30955094 Review.
References
-
- Moynahan EJ (1974) Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet 2: 399–400. - PubMed
-
- Moynahan EJ (1982) The Lancet: Acrodermatitis Enteropathica: A Lethal Inherited Human Zinc-Deficiency Disorder. Nutr Rev 40: 84–86. - PubMed
-
- Lakdawala N, Grant-Kels JM (2015) Acrodermatitis enteropathica and other nutritional diseases of the folds (intertriginous areas). Clin Dermatol 33: 414–419. doi: 10.1016/j.clindermatol.2015.04.002 - DOI - PubMed
-
- Küry S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, et al. (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 31: 239–240. doi: 10.1038/ng913 - DOI - PubMed
-
- Yuzbasiyan-Gurkan V, Bartlett E (2006) Identification of a unique splice site variant in SLC39A4 in bovine hereditary zinc deficiency, lethal trait A46: An animal model of acrodermatitis enteropathica. Genomics 88: 521–526. doi: 10.1016/j.ygeno.2006.03.018 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

