Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI)
- PMID: 29566007
- PMCID: PMC5863969
- DOI: 10.1371/journal.pone.0194180
Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI)
Abstract
Background: The inFLUenza Patient Reported Outcome (FLU-PRO) measure is a daily diary assessing signs/symptoms of influenza across six body systems: Nose, Throat, Eyes, Chest/Respiratory, Gastrointestinal, Body/Systemic, developed and tested in adults with influenza.
Objectives: This study tested the reliability, validity, and responsiveness of FLU-PRO scores in adults with influenza-like illness (ILI).
Methods: Data from the prospective, observational study used to develop and test the FLU-PRO in influenza virus positive patients were analyzed. Adults (≥18 years) presenting with influenza symptoms in outpatient settings in the US, UK, Mexico, and South America were enrolled, tested for influenza virus, and asked to complete the 37-item draft FLU-PRO daily for up to 14-days. Analyses were performed on data from patients testing negative. Reliability of the final, 32-item FLU-PRO was estimated using Cronbach's alpha (α; Day 1) and intraclass correlation coefficients (ICC; 2-day reproducibility). Convergent and known-groups validity were assessed using patient global assessments of influenza severity (PGA). Patient report of return to usual health was used to assess responsiveness (Day 1-7).
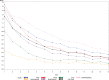
Results: The analytical sample included 220 ILI patients (mean age = 39.3, 64.1% female, 88.6% white). Sixty-one (28%) were hospitalized at some point in their illness. Internal consistency reliability (α) of FLU-PRO Total score was 0.90 and ranged from 0.72-0.86 for domain scores. Reproducibility (Day 1-2) was 0.64 for Total, ranging from 0.46-0.78 for domain scores. Day 1 FLU-PRO scores correlated (≥0.30) with the PGA (except Gastrointestinal) and were significantly different across PGA severity groups (Total: F = 81.7, p<0.001; subscales: F = 6.9-62.2; p<0.01). Mean score improvements Day 1-7 were significantly greater in patients reporting return to usual health compared with those who did not (p<0.05, Total and subscales, except Gastrointestinal and Eyes).
Conclusions: Results suggest FLU-PRO scores are reliable, valid, and responsive in adults with influenza-like illness.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention (CDC) (2016) Key Facts about Influenza (Flu) & Flu Vaccine.
-
- Centers for Disease Control and Prevention (CDC) (2016) Overview of Influenza Surveillance in the United States.
-
- Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD, et al. (2007) What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value in Health 10: S94–S105. doi: 10.1111/j.1524-4733.2007.00272.x - DOI - PubMed
-
- Revicki DA (2007) FDA draft guidance and health-outcomes research. Lancet 369: 540–542. doi: 10.1016/S0140-6736(07)60250-5 - DOI - PubMed
-
- Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, et al. (2009) Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health 12: 1075–1083. doi: 10.1111/j.1524-4733.2009.00603.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical