Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability
- PMID: 29566010
- PMCID: PMC5864000
- DOI: 10.1371/journal.pone.0194170
Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability
Abstract
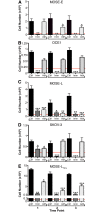
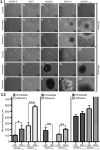
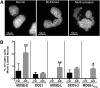
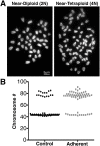
Ovarian cancer cells are exposed to physical stress in the peritoneal cavity during both tumor growth and dissemination. Ascites build-up in metastatic ovarian cancer further increases the exposure to fluid shear stress. Here, we used a murine, in vitro ovarian cancer progression model in parallel with immortalized human cells to investigate how ovarian cancer cells of increasing aggressiveness respond to [Formula: see text] of fluid-induced shear stress. This biophysical stimulus significantly reduced cell viability in all cells exposed, independent of disease stage. Fluid shear stress induced spheroid formation and altered cytoskeleton organization in more tumorigenic cell lines. While benign ovarian cells appeared to survive in higher numbers under the influence of fluid shear stress, they exhibited severe morphological changes and chromosomal instability. These results suggest that exposure of benign cells to low magnitude fluid shear stress can induce phenotypic changes that are associated with transformation and ovarian cancer progression. Moreover, exposure of tumorigenic cells to fluid shear stress enhanced anchorage-independent survival, suggesting a role in promoting invasion and metastasis.
Conflict of interest statement
Figures

References
-
- Heng HH, Stevens JB, Bremer SW, Liu G, Abdallah BY, Ye CJ. Evolutionary mechanisms and diversity in cancer. Adv Cancer Res. 2011;112:217–53. doi: 10.1016/B978-0-12-387688-1.00008-9 - DOI - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. - PubMed
-
- Howlader N KMMDBKKCLYMRJTZMALDRCHSFEJCKA Noone A M. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute. 2017;.
-
- Avraham-Chakim L, Elad D, Zaretsky U, Kloog Y, Jaffa A, Grisaru D. Fluid-flow induced wall shear stress and epithelial ovarian cancer peritoneal spreading. PLoS One. 2013;8(4):e60965 doi: 10.1371/journal.pone.0060965 - DOI - PMC - PubMed
-
- Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7(11):925–34. doi: 10.1016/S1470-2045(06)70939-1 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

