Assessment of MMP-2/-9 expression by fluorescence endoscopy for evaluation of anastomotic healing in a murine model of anastomotic leakage
- PMID: 29566031
- PMCID: PMC5863981
- DOI: 10.1371/journal.pone.0194249
Assessment of MMP-2/-9 expression by fluorescence endoscopy for evaluation of anastomotic healing in a murine model of anastomotic leakage
Abstract
Background: Disturbance of intestinal wound closure leads to insufficient anastomotic healing and is associated with considerable morbidity following colorectal resections. Matrix metalloproteinases (MMPs) play a crucial role in regulation of wound closure. Here fluorescence endoscopy was evaluated for assessment of MMP-2/-9 expression during failed intestinal anastomotic healing.
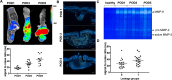
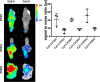
Methods: Distal colonic anastomoses were performed as a model for disturbed healing in 36 Balb/c mice. Healing was evaluated endoscopically, macroscopically, and histologically after 1, 3 and 5 days. For detection of MMP-2/-9 expression fluorescence endoscopy (FE) was used following i.v.-administration of a Cy5.5-labeled MMP-2/-9 specific tracer. FE was complemented by quantification of the fluorescence signal using the MS-FX PRO Optical Imaging System. An overall leakage score was calculated and correlated with the results of FE.
Results: With increasing incidence of anastomotic leakage from POD1 (17%) to POD5 (83%) the uptake of the MMP tracer gradually increased (signal-to-noise ratio (SNR), POD1: 17.91 ± 1.251 vs. POD3: 30.56 ± 3.03 vs. POD5: 44.8 ± 4.473, P<0.0001). Mice with defective anastomotic healing showed significantly higher uptake compared to non-defective (SNR: 37.37± 3.63 vs. 26.16± 3.635, P = 0.0369). White light endoscopy and FE allowed evaluation of anastomotic healing and visualization of mucosal MMPs in vivo. Using FE based detection of MMPs in the anastomosis, an overall positive predictive value of 71.4% and negative predictive value of 66.6% was calculated for detection of anastomotic leakage.
Conclusion: During disturbed anastomotic healing increased expression of MMP-2/-9 was observed in the anastomotic tissue. Fluorescence endoscopy for detection of MMP-2/-9 during the healing process might be a promising tool for early identification of anastomotic leakage.
Conflict of interest statement
Figures



Similar articles
-
Detection of Early Murine Colorectal Cancer by MMP-2/-9-Guided Fluorescence Endoscopy.Inflamm Bowel Dis. 2016 Jan;22(1):82-91. doi: 10.1097/MIB.0000000000000605. Inflamm Bowel Dis. 2016. PMID: 26457379
-
Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon.Int J Colorectal Dis. 2017 Sep;32(9):1277-1284. doi: 10.1007/s00384-017-2857-x. Epub 2017 Jul 17. Int J Colorectal Dis. 2017. PMID: 28717842
-
Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery.Surgery. 2005 Feb;137(2):229-34. doi: 10.1016/j.surg.2004.07.011. Surgery. 2005. PMID: 15674206
-
Postoperative non-steroidal anti-inflammatory drugs and colorectal anastomotic leakage. NSAIDs and anastomotic leakage.Dan Med J. 2012 Mar;59(3):B4420. Dan Med J. 2012. PMID: 22381097 Review.
-
Matrix metalloproteinase-9 in relation to patients with complications after colorectal surgery: a systematic review.Int J Colorectal Dis. 2021 Jan;36(1):1-10. doi: 10.1007/s00384-020-03724-6. Epub 2020 Aug 31. Int J Colorectal Dis. 2021. PMID: 32865714 Free PMC article.
Cited by
-
Innovative approaches for induction of gastrointestinal anastomotic healing: an update on experimental and clinical aspects.Langenbecks Arch Surg. 2021 Jun;406(4):971-980. doi: 10.1007/s00423-020-01957-1. Epub 2020 Aug 15. Langenbecks Arch Surg. 2021. PMID: 32803330 Free PMC article. Review.
-
Multilayered Human Skeletal Muscle Myoblast Sheets Promote the Healing Process After Colonic Anastomosis in Rats.Cell Transplant. 2021 Jan-Dec;30:9636897211009559. doi: 10.1177/09636897211009559. Cell Transplant. 2021. PMID: 33880968 Free PMC article.
-
Intestinal anastomotic healing models during experimental colitis.Int J Colorectal Dis. 2021 Oct;36(10):2247-2259. doi: 10.1007/s00384-021-04014-5. Epub 2021 Aug 28. Int J Colorectal Dis. 2021. PMID: 34455473 Free PMC article.
-
Stem cell therapy applied for digestive anastomosis: Current state and future perspectives.World J Stem Cells. 2022 Jan 26;14(1):117-141. doi: 10.4252/wjsc.v14.i1.117. World J Stem Cells. 2022. PMID: 35126832 Free PMC article.
-
Effects of the Folk Medicinal Plant Extract Ankaferd BloodStopper on the Healing of Colon Anastomosis: An Experimental Study in a Rat Model.Sisli Etfal Hastan Tip Bul. 2019 Jun 24;53(2):154-159. doi: 10.14744/SEMB.2019.98965. eCollection 2019. Sisli Etfal Hastan Tip Bul. 2019. PMID: 32377075 Free PMC article.
References
-
- Rijcken E, Sachs L, Fuchs T, Spiegel HU, Neumann PA (2014) Growth factors and gastrointestinal anastomotic healing. The Journal of surgical research 187:202–210 doi: 10.1016/j.jss.2013.10.013 - DOI - PubMed
-
- Kim SH, Son SY, Park YS, Ahn SH, Park DJ, Kim HH (2015) Risk Factors for Anastomotic Leakage: A Retrospective Cohort Study in a Single Gastric Surgical Unit. J Gastric Cancer 15:167–175 doi: 10.5230/jgc.2015.15.3.167 - DOI - PMC - PubMed
-
- Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208:269–278 doi: 10.1016/j.jamcollsurg.2008.10.015 - DOI - PubMed
-
- Agren MS, Jorgensen LN, Delaisse JM (2004) Matrix metalloproteinases and colon anastomosis repair: a new indication for pharmacological inhibition? Mini reviews in medicinal chemistry 4:769–778 - PubMed
-
- Agren MS, Andersen TL, Mirastschijski U, Syk I, Schiodt CB, Surve V, Lindebjerg J, Delaisse JM (2006) Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery 140:72–82 doi: 10.1016/j.surg.2005.12.013 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

