MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells
- PMID: 29566060
- PMCID: PMC5864050
- DOI: 10.1371/journal.pone.0194868
MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells
Abstract
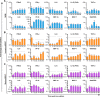
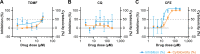
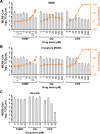
Middle East respiratory syndrome coronavirus (MERS-CoV) presents an emerging threat to public health worldwide by causing severe respiratory disease in humans with high virulence and case fatality rate (about 35%) since 2012. Little is known about the pathogenesis and innate antiviral response in primary human monocyte-derived macrophages (MDMs) and dendritic cells (MDDCs) upon MERS-CoV infection. In this study, we assessed MERS-CoV replication as well as induction of inflammatory cytokines and chemokines in MDMs and immature and mature MDDCs. Immature MDDCs and MDMs were permissive for MERS-CoV infection, while mature MDDCs were not, with stimulation of proinflammatory cytokine and chemokine upregulation in MDMs, but not in MDDCs. To further evaluate the antiviral activity of well-defined drugs in primary antigen presenting cells (APCs), three compounds (chloroquine, chlorpromazine and toremifine), each with broad-spectrum antiviral activity in immortalized cell lines, were evaluated in MDMs and MDDCs to determine their antiviral effect on MERS-CoV infection. While chloroquine was not active in these primary cells, chlorpromazine showed strong anti-MERS-CoV activity, but it was associated with high cytotoxicity narrowing the potential window for drug utilization. Unlike in established cells, toremifene had marginal activity when tested in antigen presenting cells, with high apparent cytotoxicity, also limiting its potential as a therapeutic option. These results demonstrate the value of testing drugs in primary cells, in addition to established cell lines, before initiating preclinical or clinical studies for MERS treatment and the importance of carefully assessing cytotoxicity in drug screen assays. Furthermore, these studies also highlight the role of APCs in stimulating a robust protective immune response to MERS-CoV infection.
Conflict of interest statement
Figures


References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–20. Epub 2012/10/19. doi: 10.1056/NEJMoa1211721 . - DOI - PubMed
-
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. Journal of virology. 2013;87(14):7790–2. Epub 2013/05/17. doi: 10.1128/JVI.01244-13 ; PubMed Central PMCID: PMCPmc3700179. - DOI - PMC - PubMed
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV)—Saudi Arabia Disease outbreak news. Geneva, CH: World Health Organization; 2017. [cited 2017 11 September]. Available from: http://www.who.int/csr/don/6-september-2017-mers-saudi-arabia/en/.
-
- Almario CV, Chey W, Kaung A, Whitman C, Fuller G, Reid M, et al. Computer-generated vs. physician-documented history of present illness (HPI): results of a blinded comparison. Am J Gastroenterol. 2015;110(1):170–9. doi: 10.1038/ajg.2014.356 ; PubMed Central PMCID: PMC4289091. - DOI - PMC - PubMed
-
- Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–5. doi: 10.1016/S1473-3099(13)70690-X . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

