A truncated PPAR gamma 2 localizes to mitochondria and regulates mitochondrial respiration in brown adipocytes
- PMID: 29566074
- PMCID: PMC5864067
- DOI: 10.1371/journal.pone.0195007
A truncated PPAR gamma 2 localizes to mitochondria and regulates mitochondrial respiration in brown adipocytes
Abstract
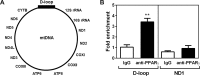
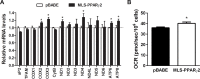
Peroxisome proliferator-activated receptor gamma (PPARγ) is a key regulator of brown adipocyte differentiation and thermogenesis. The PPARγ gene produces two isoforms, PPARγ1 and PPARγ2. PPARγ2 is identical to PPARγ1 except for additional 30 amino acids present in the N-terminus of PPARγ2. Here we report that the C-terminally truncated form of PPARγ2 is predominantly present in the mitochondrial matrix of brown adipocytes and that it binds to the D-loop region of mitochondrial DNA (mtDNA), which contains the promoter for mitochondrial electron transport chain (ETC) genes. Expression of mitochondrially targeted MLS-PPARγ2 in brown adipocytes increases mtDNA-encoded ETC gene expression concomitant with enhanced mitochondrial respiration. These results suggest that direct regulation of mitochondrially encoded ETC gene expression by mitochondrial PPARγ2, in part, underlies the isoform-specific role for PPARγ2 in brown adipocytes.
Conflict of interest statement
Figures



Similar articles
-
An unexpected role for the transcriptional coactivator isoform NT-PGC-1α in the regulation of mitochondrial respiration in brown adipocytes.J Biol Chem. 2017 Jun 16;292(24):9958-9966. doi: 10.1074/jbc.M117.778373. Epub 2017 May 4. J Biol Chem. 2017. PMID: 28473468 Free PMC article.
-
Noradrenaline represses PPAR (peroxisome-proliferator-activated receptor) gamma2 gene expression in brown adipocytes: intracellular signalling and effects on PPARgamma2 and PPARgamma1 protein levels.Biochem J. 2004 Sep 1;382(Pt 2):597-606. doi: 10.1042/BJ20031622. Biochem J. 2004. PMID: 15193150 Free PMC article.
-
Set7/9, a methyltransferase, regulates the thermogenic program during brown adipocyte differentiation through the modulation of p53 acetylation.Mol Cell Endocrinol. 2016 Aug 15;431:46-53. doi: 10.1016/j.mce.2016.04.022. Epub 2016 Apr 29. Mol Cell Endocrinol. 2016. PMID: 27132805
-
Brown vs white adipocytes: the PPARgamma coregulator story.FEBS Lett. 2010 Aug 4;584(15):3250-9. doi: 10.1016/j.febslet.2010.06.035. Epub 2010 Jun 30. FEBS Lett. 2010. PMID: 20600006 Review.
-
Meaningful respirometric measurements of UCP1-mediated thermogenesis.Biochimie. 2017 Mar;134:56-61. doi: 10.1016/j.biochi.2016.12.005. Epub 2016 Dec 14. Biochimie. 2017. PMID: 27986537 Review.
Cited by
-
PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders.Pharmacol Ther. 2021 Mar;219:107705. doi: 10.1016/j.pharmthera.2020.107705. Epub 2020 Oct 9. Pharmacol Ther. 2021. PMID: 33039420 Free PMC article. Review.
-
Human Breast Cancer Xenograft Model Implicates Peroxisome Proliferator-activated Receptor Signaling as Driver of Cancer-induced Muscle Fatigue.Clin Cancer Res. 2019 Apr 1;25(7):2336-2347. doi: 10.1158/1078-0432.CCR-18-1565. Epub 2018 Dec 17. Clin Cancer Res. 2019. PMID: 30559167 Free PMC article.
-
PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue.Int J Mol Sci. 2021 Aug 20;22(16):8974. doi: 10.3390/ijms22168974. Int J Mol Sci. 2021. PMID: 34445679 Free PMC article. Review.
-
Mito-Nuclear Communication in Hepatocellular Carcinoma Metabolic Rewiring.Cells. 2019 May 5;8(5):417. doi: 10.3390/cells8050417. Cells. 2019. PMID: 31060333 Free PMC article. Review.
-
Molecular mechanisms detected in yak lung tissue via transcriptome-wide analysis provide insights into adaptation to high altitudes.Sci Rep. 2021 Apr 8;11(1):7786. doi: 10.1038/s41598-021-87420-7. Sci Rep. 2021. PMID: 33833362 Free PMC article.
References
-
- She H, Yang Q, Shepherd K, Smith Y, Miller G, Testa C, et al. Direct regulation of complex I by mitochondrial MEF2D is disrupted in a mouse model of Parkinson disease and in human patients. J Clin Invest. 2011;121(3):930–40. doi: 10.1172/JCI43871 - DOI - PMC - PubMed
-
- De Rasmo D, Signorile A, Roca E, Papa S. cAMP response element-binding protein (CREB) is imported into mitochondria and promotes protein synthesis. FEBS J. 2009;276(16):4325–33. doi: 10.1111/j.1742-4658.2009.07133.x - DOI - PubMed
-
- Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci U S A. 2005;102(39):13915–20. doi: 10.1073/pnas.0502878102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

