The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: A systematic review and meta-analysis
- PMID: 29566078
- PMCID: PMC5864049
- DOI: 10.1371/journal.pone.0194733
The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: A systematic review and meta-analysis
Abstract
Objectives: To compare clinical outcomes of concurrent chemoradiotherapy (CCRT) with those of radiotherapy alone for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy (IMRT) era.
Materials and methods: We comprehensively searched PubMed, Embase, and the Cochrane Library to identify eligible studies. Overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), locoregional recurrence-free survival (LRRFS) with hazard ratios (HRs), and toxicities with odd ratios (ORs) were analyzed.
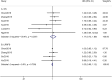
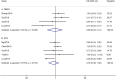
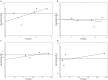
Results: A total of seven studies met the criteria, with 1302 patients who were treated with IMRT alone or IMRT plus concurrent chemotherapy. No significant survival benefit was shown by CCRT regardless of OS (HR = 1.17, 95% CI 0.73-1.89, P = 0.508), PFS (HR = 0.76, 95% CI 0.38-1.50, P = 0.430), DMFS (HR = 0.89, 95% CI 0.33-2.41, P = 0.816), or LRRFS (HR = 1.03, 95% CI 0.95-1.12, P = 0.498). Additionally, CCRT notably increased the risk of acute grade 3-4 leukopenia (OR = 4.432, 95% CI 2.195-8.952, P < 0.001), compared to IMRT alone.
Conclusion: Adding concurrent chemotherapy to IMRT led to no survival benefit and increased acute toxicity reactions for stage II nasopharyngeal carcinoma.
Conflict of interest statement
Figures
Similar articles
-
Induction chemotherapy plus IMRT alone versus induction chemotherapy plus IMRT-based concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study.J Cancer Res Clin Oncol. 2019 Jul;145(7):1857-1864. doi: 10.1007/s00432-019-02925-z. Epub 2019 May 6. J Cancer Res Clin Oncol. 2019. PMID: 31062162 Free PMC article.
-
Concurrent chemoradiotherapy versus radiotherapy alone for stage II nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy.Eur Arch Otorhinolaryngol. 2023 Jul;280(7):3097-3106. doi: 10.1007/s00405-023-07943-9. Epub 2023 Apr 20. Eur Arch Otorhinolaryngol. 2023. PMID: 37079074
-
Comparison of TPF and PF induction chemotherapy combined with cisplatin concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: A systematic review and meta-analysis.Medicine (Baltimore). 2025 Jan 17;104(3):e41278. doi: 10.1097/MD.0000000000041278. Medicine (Baltimore). 2025. PMID: 39833074 Free PMC article.
-
A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma.Ann Oncol. 2015 Jan;26(1):205-211. doi: 10.1093/annonc/mdu507. Epub 2014 Oct 29. Ann Oncol. 2015. PMID: 25355717
-
Intensity-modulated radiotherapy alone compared with intensity-modulated radiotherapy plus concurrent chemotherapy in intermediate-risk nasopharyngeal carcinoma : A prospective multicenter phase II trial.Strahlenther Onkol. 2024 Oct;200(10):867-875. doi: 10.1007/s00066-024-02201-1. Epub 2024 Feb 7. Strahlenther Onkol. 2024. PMID: 38324078 Clinical Trial.
Cited by
-
Current Role of Chemotherapy in Nonmetastatic Nasopharyngeal Cancer.J Oncol. 2018 Oct 1;2018:3725837. doi: 10.1155/2018/3725837. eCollection 2018. J Oncol. 2018. PMID: 30364069 Free PMC article. Review.
-
Retrospective analysis of 1539 nasopharyngeal carcinoma cases: chemotherapy should not be excluded for non-Asian patients with T1-2N1M0 stage.Front Oncol. 2025 Jan 17;14:1529136. doi: 10.3389/fonc.2024.1529136. eCollection 2024. Front Oncol. 2025. PMID: 39896187 Free PMC article.
-
Symptom clusters after chemoradiotherapy in discharged nasopharyngeal carcinoma patients.Front Oncol. 2023 Jun 14;13:920889. doi: 10.3389/fonc.2023.920889. eCollection 2023. Front Oncol. 2023. PMID: 37388231 Free PMC article.
-
A Systematic Review and Meta-Analysis of Studies Comparing Concurrent Chemoradiotherapy With Radiotherapy Alone in the Treatment of Stage II Nasopharyngeal Carcinoma.Front Oncol. 2022 Jul 12;12:843675. doi: 10.3389/fonc.2022.843675. eCollection 2022. Front Oncol. 2022. PMID: 35903695 Free PMC article.
-
The Role of Genetic Pathways in the Development of Chemoradiation Resistance in Nasopharyngeal Carcinoma (NPC) Patients.Genes (Basel). 2021 Nov 21;12(11):1835. doi: 10.3390/genes12111835. Genes (Basel). 2021. PMID: 34828441 Free PMC article. Review.
References
-
- Cao S-M, Simons MJ, Qian C-N. The prevalence and prevention of nasopharyngeal carcinoma in China. Chinese Journal of Cancer. 2011;30(2):114–9. doi: 10.5732/cjc.010.10377 - DOI - PMC - PubMed
-
- Chang ET, Adami HO. The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiology Biomarkers & Prevention. 2006;15(10):1765–77. doi: 10.1158/1055-9965.EPI-06-0353 - DOI - PubMed
-
- Chan AT, Gregoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii83–5. Epub 2012/11/20. doi: 10.1093/annonc/mds266 . - DOI - PubMed
-
- NCCN Guidelines Version 2.2017 Head and Neck Cancers. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
-
- Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent Chemoradiotherapy vs Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: Phase III Randomized Trial. Journal of the National Cancer Institute. 2011;103(23):1761–70. doi: 10.1093/jnci/djr432 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous