PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice
- PMID: 29566098
- PMCID: PMC5882172
- DOI: 10.1371/journal.ppat.1006933
PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice
Abstract
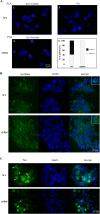
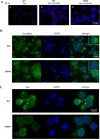
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATLL), an aggressive malignant proliferation of activated CD4+ T lymphocytes. The viral Tax oncoprotein is critically involved in both HTLV-1-replication and T-cell proliferation, a prerequisite to the development of ATLL. In this study, we investigated the in vivo contribution of the Tax PDZ domain-binding motif (PBM) to the lymphoproliferative process. To that aim, we examined T-cell proliferation in humanized mice (hu-mice) carrying a human hemato-lymphoid system infected with either a wild type (WT) or a Tax PBM-deleted (ΔPBM) provirus. We observed that the frequency of CD4+ activated T-cells in the peripheral blood and in the spleen was significantly higher in WT than in ΔPBM hu-mice. Likewise, human T-cells collected from WT hu-mice and cultivated in vitro in presence of interleukin-2 were proliferating at a higher level than those from ΔPBM animals. We next examined the association of Tax with the Scribble PDZ protein, a prominent regulator of T-cell polarity, in human T-cells analyzed either after ex vivo isolation or after in vitro culture. We confirmed the interaction of Tax with Scribble only in T-cells from the WT hu-mice. This association correlated with the presence of both proteins in aggregates at the leading edge of the cells and with the formation of long actin filopods. Finally, data from a comparative genome-wide transcriptomic analysis suggested that the PBM-PDZ association is implicated in the expression of genes regulating proliferation, apoptosis and cytoskeletal organization. Collectively, our findings suggest that the Tax PBM is an auxiliary motif that contributes to the sustained growth of HTLV-1 infected T-cells in vivo and in vitro and is essential to T-cell immortalization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo.Blood. 2006 Mar 1;107(5):1980-8. doi: 10.1182/blood-2005-03-1333. Epub 2005 Nov 1. Blood. 2006. PMID: 16263794 Free PMC article.
-
Human T-cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor Scribble through the PDZ domain-binding motif dependent and independent interaction.Virus Genes. 2008 Oct;37(2):231-40. doi: 10.1007/s11262-008-0259-4. Epub 2008 Jul 26. Virus Genes. 2008. PMID: 18661220
-
The PDZ domain binding motif (PBM) of human T-cell leukemia virus type 1 Tax can be substituted by heterologous PBMs from viral oncoproteins during T-cell transformation.Virus Genes. 2010 Apr;40(2):193-9. doi: 10.1007/s11262-009-0447-x. Virus Genes. 2010. PMID: 20069350
-
HTLV-1 Tax and adult T-cell leukemia.Front Biosci. 2007 Jan 1;12:1496-507. doi: 10.2741/2163. Front Biosci. 2007. PMID: 17127397 Review.
-
HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation.Pathogens. 2024 Jan 19;13(1):87. doi: 10.3390/pathogens13010087. Pathogens. 2024. PMID: 38276160 Free PMC article. Review.
Cited by
-
Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade.Blood. 2019 Oct 24;134(17):1406-1414. doi: 10.1182/blood.2019002038. Blood. 2019. PMID: 31467059 Free PMC article. Clinical Trial.
-
Large-scale integrative analysis of juvenile idiopathic arthritis for new insight into its pathogenesis.Arthritis Res Ther. 2024 Feb 10;26(1):47. doi: 10.1186/s13075-024-03280-2. Arthritis Res Ther. 2024. PMID: 38336809 Free PMC article.
-
Viral subversion of the cell polarity regulator Scribble.Biochem Soc Trans. 2023 Feb 27;51(1):415-426. doi: 10.1042/BST20221067. Biochem Soc Trans. 2023. PMID: 36606695 Free PMC article. Review.
-
Molecular basis of Tick Born encephalitis virus NS5 mediated subversion of apico-basal cell polarity signalling.Biochem J. 2022 Jun 30;479(12):1303-1315. doi: 10.1042/BCJ20220037. Biochem J. 2022. PMID: 35670457 Free PMC article.
-
Absence of accessory genes in a divergent simian T-lymphotropic virus type 1 isolated from a bonnet macaque (Macaca radiata).PLoS Negl Trop Dis. 2019 Jul 8;13(7):e0007521. doi: 10.1371/journal.pntd.0007521. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31283766 Free PMC article.
References
-
- Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517–26. doi: 10.1016/S1470-2045(14)70202-5 . - DOI - PubMed
-
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–80. doi: 10.1038/nrc2111 . - DOI - PubMed
-
- Baydoun HH, Bai XT, Shelton S, Nicot C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS One. 2012;7(8):e42226 doi: 10.1371/journal.pone.0042226 ; PubMed Central PMCID: PMCPMC3423393. - DOI - PMC - PubMed
-
- Haoudi A, Semmes OJ. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology. 2003;305(2):229–39. . - PubMed
-
- Matsuoka M, Jeang KT. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy. Oncogene. 2011;30(12):1379–89. doi: 10.1038/onc.2010.537 ; PubMed Central PMCID: PMCPMC3413891. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

