Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: A Clinical Trial
- PMID: 29566104
- PMCID: PMC5885212
- DOI: 10.1001/jamaoncol.2018.0060
Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: A Clinical Trial
Abstract
Importance: Cotargeting the mammalian target of rapamycin pathway and estrogen receptor may prevent or delay endocrine resistance in patients receiving first-line treatment for advanced breast cancer.
Objective: To investigate the combination of everolimus plus endocrine therapy in first-line and second-line treatment settings for postmenopausal women with estrogen receptor-positive, human epidermal growth receptor 2-negative advanced breast cancer.
Design, setting, and participants: In the multicenter, open-label, single-arm, phase 2 BOLERO-4 (Breast Cancer Trials of Oral Everolimus) clinical trial, 245 patients were screened for eligibility; 202 were enrolled between March 7, 2013, and December 17, 2014. A median follow-up of 29.5 months had been achieved by the data cutoff date (December 17, 2016).
Interventions: Patients received first-line treatment with everolimus, 10 mg/d, plus letrozole, 2.5 mg/d. Second-line treatment with everolimus, 10 mg/d, plus exemestane, 25 mg/d, was offered at the investigator's discretion upon initial disease progression.
Main outcomes and measures: The primary end point was investigator-assessed progression-free survival in the first-line setting per Response Evaluation Criteria in Solid Tumors, version 1.0. Safety was assessed in patients who received at least 1 dose of study medication and at least 1 postbaseline safety assessment.
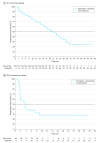
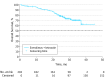
Results: A total of 202 women treated in the first-line setting had a median age of 64.0 years (interquartile range, 58.0-70.0 years) with metastatic (194 [96.0%]) or locally advanced (8 [4.0%]) breast cancer. Median progression-free survival was 22.0 months (95% CI, 18.1-25.1 months) with everolimus and letrozole. Median overall survival was not reached; 24-month estimated overall survival rate was 78.7% (95% CI, 72.1%-83.9%). Fifty patients started second-line treatment; median progression-free survival was 3.7 months (95% CI, 1.9-7.4 months). No new safety signals were observed. In the first-line setting, the most common all-grade adverse event was stomatitis (139 [68.8%]); the most common grade 3 to 4 adverse event was anemia (21 [10.4%]). In the second-line setting, the most common adverse events were stomatitis and decreased weight (10 [20.0%] each); the most common grade 3 to 4 adverse event was hypertension (5 [10.0%]). There were 50 (24.8%) deaths overall during the study; 40 were due to study indication (breast cancer).
Conclusions and relevance: The results of this trial add to the existing body of evidence suggesting that everolimus plus endocrine therapy is a good first-line treatment option for postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer.
Trial registration: clinicaltrials.gov Identifier: NCT01698918.
Conflict of interest statement
Figures
References
-
- NCCN Clinical Practice Guidelines in Oncology Breast Cancer. V2.2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 27, 2017.
-
- Gnant M. Overcoming endocrine resistance in breast cancer: importance of mTOR inhibition. Expert Rev Anticancer Ther. 2012;12(12):1579-1589. - PubMed
-
- Bachelot T, Bourgier C, Cropet C, et al. . Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718-2724. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

