Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction
- PMID: 29566124
- PMCID: PMC5967544
- DOI: 10.1093/cvr/cvy067
Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction
Abstract
Aims: Previous studies have demonstrated improved cardiac function following myocardial infarction (MI) after administration of endothelial progenitor cells (EPCs) into ischaemic myocardium. A growing body of literature supports paracrine effectors, including extracellular vesicles (EVs), as the main mediators of the therapeutic benefits of EPCs. The direct use of paracrine factors is an attractive strategy that harnesses the effects of cell therapy without concerns of cell engraftment or viability. We aim to reproduce the beneficial effects of EPC treatment through delivery of EPC-derived EVs within a shear-thinning gel (STG) for precise localization and sustained delivery.
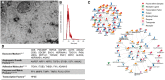
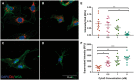
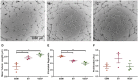
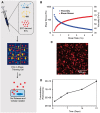
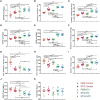
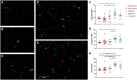
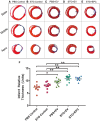
Methods and results: EVs were harvested from EPCs isolated from adult male Rattus norvegicus (Wistar) rats and characterized by electron microscopy, nanoparticle tracking analysis (NTA), and mass spectrometry. EVs were incorporated into the STG and injected at the border zone in rat models of MI. Haemodynamic function, angiogenesis, and myocardial remodelling were analyzed in five groups: phosphate buffered saline (PBS) control, STG control, EVs in PBS, EVs in STG, and EPCs in STG. Electron microscopy and NTA of EVs showed uniform particles of 50-200 nm. EV content analysis revealed several key angiogenic mediators. EV uptake by endothelial cells was confirmed and followed by robust therapeutic angiogenesis. In vivo animal experiments demonstrated that delivery of EVs within the STG resulted in increased peri-infarct vascular proliferation, preservation of ventricular geometry, and improved haemodynamic function post-MI.
Conclusions: EPC-derived EVs delivered into ischaemic myocardium via an injectable hydrogel enhanced peri-infarct angiogenesis and myocardial haemodynamics in a rat model of MI. The STG greatly increased therapeutic efficiency and efficacy of EV-mediated myocardial preservation.
Figures

Similar articles
-
Delayed delivery of endothelial progenitor cell-derived extracellular vesicles via shear thinning gel improves postinfarct hemodynamics.J Thorac Cardiovasc Surg. 2020 May;159(5):1825-1835.e2. doi: 10.1016/j.jtcvs.2019.06.017. Epub 2019 Jun 18. J Thorac Cardiovasc Surg. 2020. PMID: 31353103 Free PMC article.
-
Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium.J Thorac Cardiovasc Surg. 2015 Nov;150(5):1268-76. doi: 10.1016/j.jtcvs.2015.07.035. Epub 2015 Jul 17. J Thorac Cardiovasc Surg. 2015. PMID: 26293548 Free PMC article.
-
Delivery of progenitor cells with injectable shear-thinning hydrogel maintains geometry and normalizes strain to stabilize cardiac function after ischemia.J Thorac Cardiovasc Surg. 2019 Apr;157(4):1479-1490. doi: 10.1016/j.jtcvs.2018.07.117. Epub 2018 Nov 14. J Thorac Cardiovasc Surg. 2019. PMID: 30579534 Free PMC article.
-
Angiogenesis after acute myocardial infarction.Cardiovasc Res. 2021 Apr 23;117(5):1257-1273. doi: 10.1093/cvr/cvaa287. Cardiovasc Res. 2021. PMID: 33063086 Review.
-
Endothelial progenitor cells in neovascularization of infarcted myocardium.J Mol Cell Cardiol. 2008 Oct;45(4):530-44. doi: 10.1016/j.yjmcc.2008.08.003. Epub 2008 Aug 22. J Mol Cell Cardiol. 2008. PMID: 18755197 Free PMC article. Review.
Cited by
-
Exploring the role of polymers to overcome ongoing challenges in the field of extracellular vesicles.J Extracell Vesicles. 2023 Dec;12(12):e12386. doi: 10.1002/jev2.12386. J Extracell Vesicles. 2023. PMID: 38050832 Free PMC article. Review.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment.Stem Cell Res Ther. 2022 Jun 7;13(1):238. doi: 10.1186/s13287-022-02921-0. Stem Cell Res Ther. 2022. PMID: 35672766 Free PMC article. Review.
-
Endothelial Progenitor Cells: New Targets for Therapeutics for Inflammatory Conditions With High Cardiovascular Risk.Front Med (Lausanne). 2018 Jul 10;5:200. doi: 10.3389/fmed.2018.00200. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30042945 Free PMC article. Review.
-
Regulation of Endothelial Progenitor Cell Functions in Ischemic Heart Disease: New Therapeutic Targets for Cardiac Remodeling and Repair.Front Cardiovasc Med. 2022 May 23;9:896782. doi: 10.3389/fcvm.2022.896782. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35677696 Free PMC article. Review.
References
-
- Murphy SL, Xu J, Kochanek KD.. Deaths: final data for 2010. Natl Vital Stat Rep 2013;61:1–117. - PubMed
-
- Head SJ, Mack MJ, Holmes DR Jr, Mohr FW, Morice MC, Serruys PW, Kappetein AP.. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg 2012;41:535–541. - PubMed
-
- Sutton MG, Sharpe N.. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000;101:2981–2988. - PubMed
-
- Sheikh AY, Huber BC, Narsinh KH, Spin JM, van der Bogt K, de Almeida PE, Ransohoff KJ, Kraft DL, Fajardo G, Ardigo D, Ransohoff J, Bernstein D, Fischbein MP, Robbins RC, Wu JC.. In vivo functional and transcriptional profiling of bone marrow stem cells after transplantation into ischemic myocardium. Arterioscler Thromb Vasc Biol 2012;32:92–102. - PMC - PubMed
-
- Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, Fazel S, Weisel RD, Yau T, Wu JC, Li RK.. Tracking cardiac engraftment and distribution of implanted bone marrow cells: comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg 2009;137:1225–1233.e1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

