Allelic Variation in a Single Genomic Region Alters the Microbiome of the Snail Biomphalaria glabrata
- PMID: 29566237
- PMCID: PMC6022664
- DOI: 10.1093/jhered/esy014
Allelic Variation in a Single Genomic Region Alters the Microbiome of the Snail Biomphalaria glabrata
Abstract
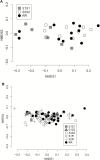
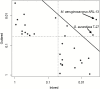
Freshwater snails are the intermediate hosts for numerous parasitic worms which can have negative consequences for human health and agriculture. Understanding the transmission of these diseases requires a more complete characterization of the immunobiology of snail hosts. This includes the characterization of its microbiome and genetic factors which may interact with this important commensal community. Allelic variation in the Guadeloupe resistance complex (GRC) genomic region of Guadeloupean Biomphalaria glabrata influences their susceptibility to schistosome infection and may have other roles in the snail immune response. In the present study, we examined whether a snail's GRC genotype has a role in shaping the bacterial diversity and composition present on or in whole snails. We show that the GRC haplotype, including the resistant genotype, has a significant effect on the diversity of bacterial species present in or on whole snails, including the relative abundances of Gemmatimonas aurantiaca and Micavibrio aeruginosavorus. These findings support the hypothesis that the GRC region is likely involved in pathways that can modify the microbial community of these snails and may have more immune roles in B. glabrata than originally believed. This is also one of few examples in which allelic variation at a particular locus has been shown to affect the microbiome in any species.
Figures
References
-
- Allan ERO, Gourbal B, Dores CB, Portet A, Bayne CJ, Blouin MS, 2017c. Clearance of schistosome parasites by resistant genotypes at a single genomic region in Biomphalaria glabrata snails involves cellular components of the hemolymph. Int J Parasitol. Advance Access published November 12, 2017. Available from: https://doi.org/10.1016/j.ijpara.2017.08.008 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

