The DNA methylation profile of liver tumors in C3H mice and identification of differentially methylated regions involved in the regulation of tumorigenic genes
- PMID: 29566670
- PMCID: PMC5865360
- DOI: 10.1186/s12885-018-4221-0
The DNA methylation profile of liver tumors in C3H mice and identification of differentially methylated regions involved in the regulation of tumorigenic genes
Abstract
Background: C3H mice have been frequently used in cancer studies as animal models of spontaneous liver tumors and chemically induced hepatocellular carcinoma (HCC). Epigenetic modifications, including DNA methylation, are among pivotal control mechanisms of gene expression leading to carcinogenesis. Although information on somatic mutations in liver tumors of C3H mice is available, epigenetic aspects are yet to be clarified.
Methods: We performed next generation sequencing-based analysis of DNA methylation and microarray analysis of gene expression to explore genes regulated by DNA methylation in spontaneous liver tumors of C3H mice. Overlaying these data, we selected cancer-related genes whose expressions are inversely correlated with DNA methylation levels in the associated differentially methylated regions (DMRs) located around transcription start sites (TSSs) (promoter DMRs). We further assessed mutuality of the selected genes for expression and DNA methylation in human HCC using the Cancer Genome Atlas (TCGA) database.
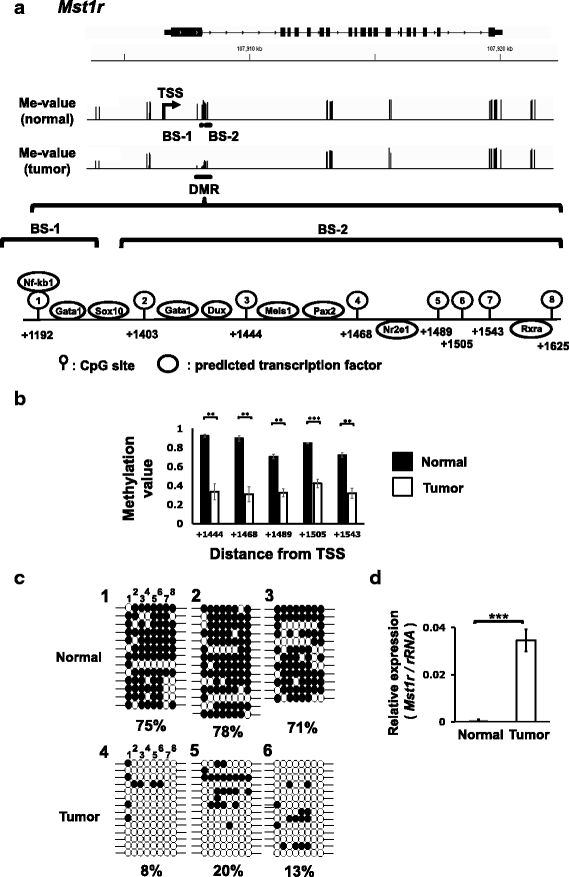
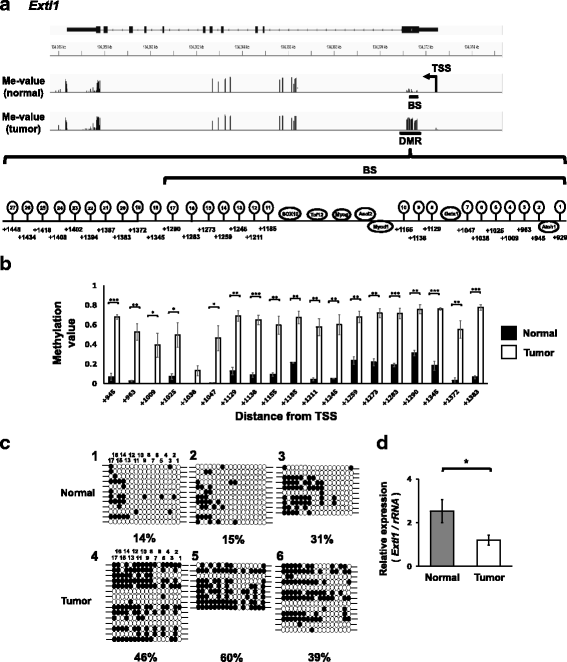
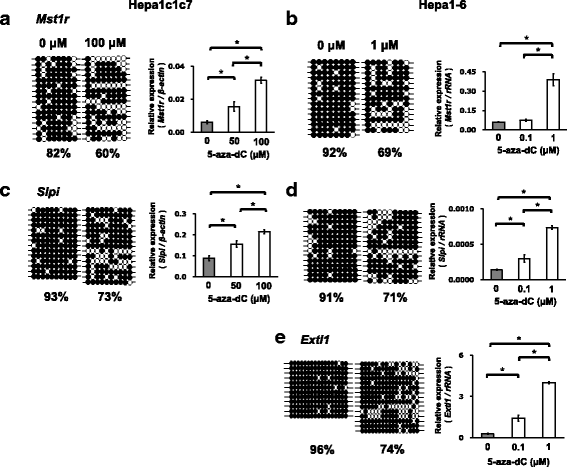
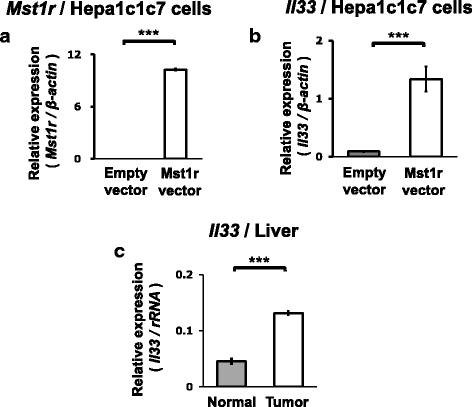
Results: We obtained data on genome-wide DNA methylation profiles in the normal and tumor livers of C3H mice. We identified promoter DMRs of genes which are reported to be related to cancer and whose expressions are inversely correlated with the DNA methylation, including Mst1r, Slpi and Extl1. The association between DNA methylation and gene expression was confirmed using a DNA methylation inhibitor 5-aza-2'-deoxycytidine (5-aza-dC) in Hepa1c1c7 cells and Hepa1-6 cells. Overexpression of Mst1r in Hepa1c1c7 cells illuminated a novel downstream pathway via IL-33 upregulation. Database search indicated that gene expressions of Mst1r and Slpi are upregulated and the TSS upstream regions are hypomethylated also in human HCC. These results suggest that DMRs, including those of Mst1r and Slpi, are involved in liver tumorigenesis in C3H mice, and also possibly in human HCC.
Conclusions: Our study clarified genome wide DNA methylation landscape of C3H mice. The data provide useful information for further epigenetic studies of mice models of HCC. The present study particularly proposed novel DNA methylation-regulated pathways for Mst1r and Slpi, which may be applied not only to mouse HCC but also to human HCC.
Keywords: 5-aza-2'-deoxycytidine; C3H mice; DNA methylation; Liver tumors; Reduced representation bisulfite sequencing (RRBS).
Conflict of interest statement
Ethics approval and consent to participate
Animal studies were permitted by the Animal Care and Use Committee of National Institute for Environmental Studies (NIES) and were performed in accordance with guideline for the Care and Use of Laboratory Animals of NIES.
The results on human HCC shown in Fig. 7 are based on existing data generated by The Cancer Genome Atlas project established by the NCI and NHGRI
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Delgado E, Okabe H, Preziosi M, Russell JO, Alvarado TF, Oertel M, Nejak-Bowen KN, Zhang Y, Monga SP. Complete response of Ctnnb1-mutated tumours to beta-catenin suppression by locked nucleic acid antisense in a mouse hepatocarcinogenesis model. J Hepatol. 2015;62(2):380–387. doi: 10.1016/j.jhep.2014.10.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

