Intrauterine resuscitation during the second stage of term labour by maternal hyperoxygenation versus conventional care: study protocol for a randomised controlled trial (INTEREST O2)
- PMID: 29566729
- PMCID: PMC5865381
- DOI: 10.1186/s13063-018-2567-x
Intrauterine resuscitation during the second stage of term labour by maternal hyperoxygenation versus conventional care: study protocol for a randomised controlled trial (INTEREST O2)
Erratum in
-
Correction to: Intrauterine resuscitation during the second stage of term labour by maternal hyperoxygenation versus conventional care: study protocol for a randomised controlled trial (INTEREST O2).Trials. 2018 Oct 23;19(1):580. doi: 10.1186/s13063-018-2963-2. Trials. 2018. PMID: 30352596 Free PMC article.
Abstract
Background: Perinatal asphyxia is, even in developed countries, one the major causes of neonatal morbidity and mortality. Therefore, if foetal distress during labour is suspected, one should try to restore foetal oxygen levels or aim for immediate delivery. However, studies on the effect of intrauterine resuscitation during labour are scarce. We designed a randomised controlled trial to investigate the effect of maternal hyperoxygenation on the foetal condition. In this study, maternal hyperoxygenation is induced for the treatment of foetal distress during the second stage of term labour.
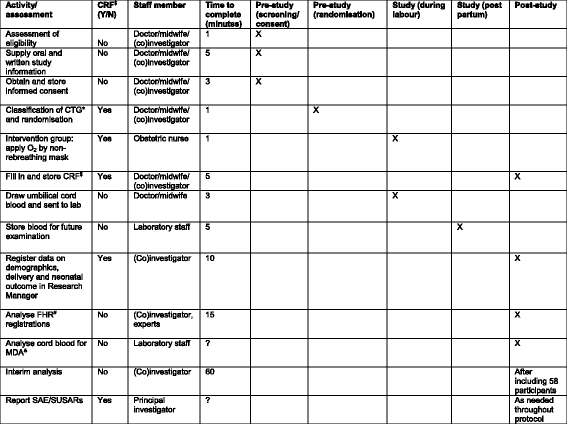
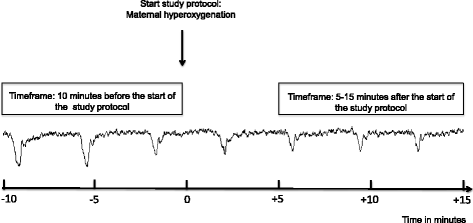
Methods/design: This study is a single-centre randomised controlled trial being performed in a tertiary hospital in The Netherlands. From among cases of a suboptimal or abnormal foetal heart rate pattern during the second stage of term labour, a total of 116 patients will be randomised to the control group, where normal care is provided, or to the intervention group, where before normal care 100% oxygen is supplied to the mother by a non-rebreathing mask until delivery. The primary outcome is change in foetal heart rate pattern. Secondary outcomes are Apgar score, mode of delivery, admission to the neonatal intensive care unit and maternal side effects. In addition, blood gas values and malondialdehyde are determined in umbilical cord blood.
Discussion: This study will be the first randomised controlled trial to investigate the effect of maternal hyperoxygenation for foetal distress during labour. This intervention should be recommended only as a treatment for intrapartum foetal distress, when improvement of the foetal condition is likely and outweighs maternal and neonatal side effects.
Trial registration: EudraCT, 2015-001654-15; registered on 3 April 2015. Dutch Trial Register, NTR5461; registered on 20 October 2015.
Keywords: Cardiotocogram; Foetal distress; Foetal heart rate; Free oxygen radicals; Intrauterine resuscitation; Maternal hyperoxygenation; Neonatal outcome; Randomised controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
This study (including amendments) was approved by the Central Committee on Research Involving Human Subjects (protocol number NL53018.000.15). The study was registered in the EudraCT database (2015-001654-15) on 3 April 2015 and in the Dutch Trial Register (NTR5461) on 20 October 2015. This published study protocol corresponds to the seventh version of the original protocol, dated 2 November 2016. The sponsor (Máxima Medical Centre Board of Management, Veldhoven, The Netherlands) approved the conduct of this study at their hospital. The study will be conducted according to the principles of the Declaration of Helsinki (64th version, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). All patients will receive oral and written information about the study and must give their written informed consent before randomisation. When handling personal data, we adhere to the Dutch Personal Data Protection Act.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The effect of intrauterine resuscitation by maternal hyperoxygenation on perinatal and maternal outcome: a randomized controlled trial.Am J Obstet Gynecol MFM. 2020 May;2(2):100102. doi: 10.1016/j.ajogmf.2020.100102. Epub 2020 Mar 21. Am J Obstet Gynecol MFM. 2020. PMID: 33345953 Clinical Trial.
-
Prevention of cerebral palsy during labour: role of foetal lactate.Arch Gynecol Obstet. 2008 Jul;278(1):17-22. doi: 10.1007/s00404-007-0531-1. Epub 2007 Dec 11. Arch Gynecol Obstet. 2008. PMID: 18071726
-
Effect of maternal hyperoxygenation on neonatal outcomes among women in labour with pathological cardiotocography: an open-label randomized controlled trial.Am J Obstet Gynecol. 2024 Apr;230(4):454.e1-454.e11. doi: 10.1016/j.ajog.2023.09.093. Epub 2023 Sep 29. Am J Obstet Gynecol. 2024. PMID: 37778675 Clinical Trial.
-
[Abnormal fetal heart rate patterns associated with different labour managements and intrauterine resuscitation techniques].J Gynecol Obstet Biol Reprod (Paris). 2008 Feb;37 Suppl 1:S56-64. doi: 10.1016/j.jgyn.2007.11.011. Epub 2008 Jan 9. J Gynecol Obstet Biol Reprod (Paris). 2008. PMID: 18187267 Review. French.
-
Intrauterine resuscitation during labor: should maternal oxygen administration be a first-line measure?Semin Fetal Neonatal Med. 2008 Dec;13(6):362-7. doi: 10.1016/j.siny.2008.04.016. Epub 2008 Jun 4. Semin Fetal Neonatal Med. 2008. PMID: 18534928 Review.
Cited by
-
Correction to: Intrauterine resuscitation during the second stage of term labour by maternal hyperoxygenation versus conventional care: study protocol for a randomised controlled trial (INTEREST O2).Trials. 2018 Oct 23;19(1):580. doi: 10.1186/s13063-018-2963-2. Trials. 2018. PMID: 30352596 Free PMC article.
References
-
- Caldeyro-Barcia R, Mendez-Bauer C, Poseiro J, Escarena L, Pose S, Bieniarz A. Control of the human fetal heart rate during labor. In: Cassels DE, editor. The heart and circulation in the newborn and infant: an international symposium presented by the Chicago Heart Association. New York: Grune & Stratton; 1966. pp. 7–36.
-
- Murray ML. Antepartal and intrapartal fetal monitoring. 3. New York: Springer; 2007.
-
- Westgate JA, Wibbens B, Bennet L, Wassink G, Parer JT, Gunn AJ. The intrapartum deceleration in center stage: a physiologic approach to the interpretation of fetal heart rate changes in labor. Am J Obstet Gynecol. 2007;197. 236:e1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

