Collaborative care for the detection and management of depression among adults with hypertension in South Africa: study protocol for the PRIME-SA randomised controlled trial
- PMID: 29566730
- PMCID: PMC5863904
- DOI: 10.1186/s13063-018-2518-6
Collaborative care for the detection and management of depression among adults with hypertension in South Africa: study protocol for the PRIME-SA randomised controlled trial
Abstract
Background: The high co-morbidity of mental disorders, particularly depression, with non-communicable diseases (NCDs) such as cardiovascular disease (CVD), is concerning given the rising burden of NCDs globally, and the role depression plays in confounding prevention and treatment of NCDs. The objective of this randomised control trial (RCT) is to determine the real-world effectiveness of strengthened depression identification and management on depression outcomes in hypertensive patients attending primary health care (PHC) facilities in South Africa (SA).
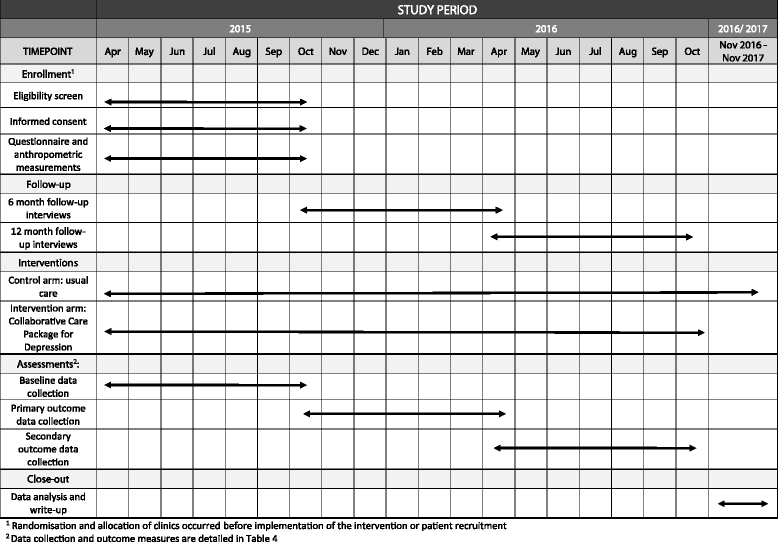
Methods/design: The study design is a pragmatic, two-arm, parallel-cluster RCT, the unit of randomisation being the clinics, with outcomes being measured for individual participants. The 20 largest eligible clinics from one district in the North West Province are enrolled in the trial. Equal numbers of hypertensive patients (n = 50) identified as having depression using the Patient Health Questionnaire (PHQ-9) are enrolled from each clinic, making up a total of 1000 participants with 500 in each arm. The nurse clinicians in the control facilities receive the standard training in Primary Care 101 (PC101), a clinical decision support tool for integrated chronic care that includes guidelines for hypertension and depression care. Referral pathways available include referrals to PHC physicians, clinical or counselling psychologists and outpatient psychiatric and psychological services. In the intervention clinics, this training is supplemented with strengthened training in the depression components of PC101 as well as training in clinical communication skills for nurse-led chronic care. Referral pathways are strengthened through the introduction of a facility-based behavioural health counsellor, trained to provide structured manualised counselling for depression and adherence counselling for all chronic conditions. The primary outcome is defined as at least 50% reduction in PHQ-9 score measured at 6 months.
Discussion: This trial should provide evidence of the real world effectiveness of strengtheneddepression identification and collaborative management on health outcomes of hypertensive patients withcomorbid depression attending PHC facilities in South Africa.
Trial registration: South African National Clinical Trial Register: SANCTR ( http://www.sanctr.gov.za/SAClinicalTrials ) (DOH-27-0916-5051). Registered on 9 April 2015. ClinicalTrials.gov : ID: NCT02425124 . Registered on 22 April 2015.
Keywords: Depression; Hypertension; Integrated health care; Low- and middle-income countries; Primary health care.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for the study was obtained from the University of KwaZulu-Natal’s Biomedical Research Ethics Committee (BREC) (reference BFC049/15); the University of Cape Town’s Faculty of Health Sciences Human Research Ethics Committee (reference HREC: 412/2011); as well as the Department of Health in the North West Province of South Africa. All amendments to the protocol are approved by BREC at the University of KwaZulu-Natal and amended on the trial registries. Given that randomisation of the clinics to the intervention and control condition was carried out prior to data collection, informed consent was only obtained from patients to participate in individual data collection and not for randomisation. Informed consent procedures have been described in detail in the recruitment section. Informed consent required that patients understood that their participation was voluntary and that they could withdraw at any stage; were required to sit for three interviews (baseline, 6 months and 12 months), and permit access to their patient records for research purposes.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Mortality GBD. Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- van Zyl S, et al. Risk-factor profiles for chronic diseases of lifestyle and metabolic syndrome in an urban and rural setting in South Africa. 2012;4:2012.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous