Tau Kinetics in Neurons and the Human Central Nervous System
- PMID: 29566794
- PMCID: PMC6137722
- DOI: 10.1016/j.neuron.2018.02.015
Tau Kinetics in Neurons and the Human Central Nervous System
Erratum in
-
Tau Kinetics in Neurons and the Human Central Nervous System.Neuron. 2018 May 16;98(4):861-864. doi: 10.1016/j.neuron.2018.04.035. Neuron. 2018. PMID: 29772204 Free PMC article. No abstract available.
Abstract
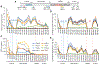
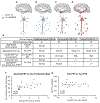
We developed stable isotope labeling and mass spectrometry approaches to measure the kinetics of multiple isoforms and fragments of tau in the human central nervous system (CNS) and in human induced pluripotent stem cell (iPSC)-derived neurons. Newly synthesized tau is truncated and released from human neurons in 3 days. Although most tau proteins have similar turnover, 4R tau isoforms and phosphorylated forms of tau exhibit faster turnover rates, suggesting unique processing of these forms that may have independent biological activities. The half-life of tau in control human iPSC-derived neurons is 6.74 ± 0.45 days and in human CNS is 23 ± 6.4 days. In cognitively normal and Alzheimer's disease participants, the production rate of tau positively correlates with the amount of amyloid plaques, indicating a biological link between amyloid plaques and tau physiology.
Keywords: Alzheimer’s disease; PET; SILK; amyloid; human; induced pluripotent stem cell; isoform; phosphorylation; positron emission tomography; production rate; stable isotope labeling kinetics; tau.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
Washington University and R.J.B. have equity ownership interest in C2N Diagnostics and may receive royalty income based on technology licensed by Washington University to C2N Diagnostics. R.J.B. receives income from C2N Diagnostics for serving on the Scientific Advisory Board, and B.W.P. receives consultation income from C2N Diagnostics. T.L.S.B. participates in research sponsored by Avid Radiopharmaceuticals, Eli Lilly, Roche, Johnson & Johnson, and Biogen. Washington University has submitted the U.S. non-provisional patent application “Methods for measuring the metabolism of CNS derived biomolecules in vivo,” serial #12/267,974 (R.J.B. and K.G.M.), and “Tau kinetics measurements,” serial #CA 2962969 (R.J.B., T.M.M., K.G.M., and C.S.). The remaining co-authors have no conflicts to disclose.
Figures


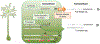
Comment in
-
Tauomics and Kinetics in Human Neurons and Biological Fluids.Neuron. 2018 Mar 21;97(6):1202-1205. doi: 10.1016/j.neuron.2018.02.030. Neuron. 2018. PMID: 29566788
References
-
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, and Hutton M (1999). Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet 8, 711–715. - PubMed
-
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, and Nishikawa S (2011). Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA 108, 14234–14239. - PMC - PubMed
-
- Barthélemy NR, Fenaille F, Hirtz C, Sergeant N, Schraen-Maschke S, Vialaret J, Buée L, Gabelle A, Junot C, Lehmann S, and Becher F (2016). Tau protein quantification in human cerebrospinal fluid by targeted mass spectrometry at high sequence coverage provides insights into its primary structure heterogeneity. J. Proteome Res 15, 667–676. - PubMed
-
- Barthélemy NR, Bateman RJ, Marin P, Becher F, Sato C, Lehmann S, and Gabelle A (2017). Tau hyperphosphorylation on T217 in cerebrospinal fluid is specifically associated to amyloid-β pathology. bioRxiv. 10.1101/226977. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- K01 AG053474/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- K01 AG046374/AG/NIA NIH HHS/United States
- R01 NS078398/NS/NINDS NIH HHS/United States
- P30 NS048056/NS/NINDS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 NS095773/NS/NINDS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

