Cancer immunotherapy using checkpoint blockade
- PMID: 29567705
- PMCID: PMC7391259
- DOI: 10.1126/science.aar4060
Cancer immunotherapy using checkpoint blockade
Abstract
The release of negative regulators of immune activation (immune checkpoints) that limit antitumor responses has resulted in unprecedented rates of long-lasting tumor responses in patients with a variety of cancers. This can be achieved by antibodies blocking the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death 1 (PD-1) pathway, either alone or in combination. The main premise for inducing an immune response is the preexistence of antitumor T cells that were limited by specific immune checkpoints. Most patients who have tumor responses maintain long-lasting disease control, yet one-third of patients relapse. Mechanisms of acquired resistance are currently poorly understood, but evidence points to alterations that converge on the antigen presentation and interferon-γ signaling pathways. New-generation combinatorial therapies may overcome resistance mechanisms to immune checkpoint therapy.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
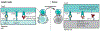


References
-
- Chambers CA, Kuhns MS, Egen JG, Allison JP, CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 19, 565–594 (2001). - PubMed
-
- Leach DR, Krummel MF, Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736. (1996). - PubMed
-
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G, Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 100, 4712–4717 (2003); published online EpubApr 15 ( - PMC - PubMed
-
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J, Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 23, 8968–8977 (2005); published online EpubDec 10 (JCO.2005.01.109 [pii] 10.1200/JCO.2005.01.109). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

