Locally translated mTOR controls axonal local translation in nerve injury
- PMID: 29567716
- PMCID: PMC6501578
- DOI: 10.1126/science.aan1053
Locally translated mTOR controls axonal local translation in nerve injury
Abstract
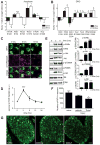
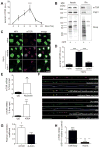
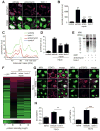
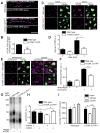
How is protein synthesis initiated locally in neurons? We found that mTOR (mechanistic target of rapamycin) was activated and then up-regulated in injured axons, owing to local translation of mTOR messenger RNA (mRNA). This mRNA was transported into axons by the cell size-regulating RNA-binding protein nucleolin. Furthermore, mTOR controlled local translation in injured axons. This included regulation of its own translation and that of retrograde injury signaling molecules such as importin β1 and STAT3 (signal transducer and activator of transcription 3). Deletion of the mTOR 3' untranslated region (3'UTR) in mice reduced mTOR in axons and decreased local translation after nerve injury. Both pharmacological inhibition of mTOR in axons and deletion of the mTOR 3'UTR decreased proprioceptive neuronal survival after nerve injury. Thus, mRNA localization enables spatiotemporal control of mTOR pathways regulating local translation and long-range intracellular signaling.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
Comment in
-
RNA targeting and translation in axons.Science. 2018 Mar 23;359(6382):1331-1332. doi: 10.1126/science.aat1498. Science. 2018. PMID: 29567693 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

