Automated decision tree to evaluate genetic abnormalities when determining prognostic risk in acute myeloid leukemia
- PMID: 29567769
- PMCID: PMC6068037
- DOI: 10.3324/haematol.2018.190926
Automated decision tree to evaluate genetic abnormalities when determining prognostic risk in acute myeloid leukemia
Figures
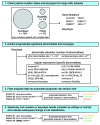


Similar articles
-
Cytogenetic heterogeneity negatively impacts outcomes in patients with acute myeloid leukemia.Haematologica. 2015 Mar;100(3):331-5. doi: 10.3324/haematol.2014.117267. Epub 2014 Dec 19. Haematologica. 2015. PMID: 25527568 Free PMC article.
-
Chromosome abnormalities at onset of complete remission are associated with worse outcome in patients with acute myeloid leukemia and an abnormal karyotype at diagnosis: CALGB 8461 (Alliance).Haematologica. 2016 Dec;101(12):1516-1523. doi: 10.3324/haematol.2016.149542. Epub 2016 Jul 28. Haematologica. 2016. PMID: 27470602 Free PMC article.
-
Association of the type of 5q loss with complex karyotype, clonal evolution, TP53 mutation status, and prognosis in acute myeloid leukemia and myelodysplastic syndrome.Genes Chromosomes Cancer. 2014 May;53(5):402-10. doi: 10.1002/gcc.22151. Epub 2014 Feb 3. Genes Chromosomes Cancer. 2014. PMID: 24493299
-
Innovative strategies for adverse karyotype acute myeloid leukemia.Curr Opin Hematol. 2017 Mar;24(2):89-98. doi: 10.1097/MOH.0000000000000318. Curr Opin Hematol. 2017. PMID: 28099271 Review.
-
Progress in acute myeloid leukemia.Clin Lymphoma Myeloma Leuk. 2015 Mar;15(3):139-51. doi: 10.1016/j.clml.2014.08.006. Epub 2014 Sep 19. Clin Lymphoma Myeloma Leuk. 2015. PMID: 25441110 Free PMC article. Review.
References
-
- Kihara R, Nagata Y, Kiyoi H, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28(8):1586–1595. - PubMed
-
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

