Human transbodies that interfere with the functions of Ebola virus VP35 protein in genome replication and transcription and innate immune antagonism
- PMID: 29568066
- PMCID: PMC5864874
- DOI: 10.1038/s41426-018-0031-3
Human transbodies that interfere with the functions of Ebola virus VP35 protein in genome replication and transcription and innate immune antagonism
Abstract
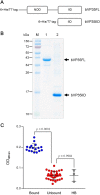
Small molecular inhibitors and passive immunization against Ebola virus disease (EVD) have been tested in animal models, including rodents and non-human primates, as well as in clinical trials. Nevertheless, there is currently no Food and Drug Administration (FDA)-approved therapy, and alternative strategies must be pursued. The aim of this study was to produce cell-penetrable human single-chain antibodies (transbodies) that are able to interfere with the activities of interferon inhibitory domain (IID) of the VP35 protein, a multifunctional virulence factor of Ebola virus (EBOV). We speculated that effective VP35-IID-specific transbodies could inspire further studies to identify an alternative to conventional antibody therapies. Phage display technology was used to generate Escherichia coli-derived human single-chain antibodies (HuscFvs) that bind to IID. HuscFvs were linked to nona-arginine (R9) to make them cell penetrable. Transbodies of transformed E. coli clones 13 and 3, which were predicted to interact with first basic patch residues (R9-HuscFv13), central basic patch, and end-cap residues (R9-HuscFv3), effectively inhibited EBOV minigenome activity. Transbodies of E. coli clones 3 and 8 antagonized VP35-mediated interferon suppression in VP35-transduced cells. We postulate that these transbodies formed an interface contact with the IID central basic patch, end-cap, and/or residues that are important for IID multimeric formation for dsRNA binding. These transbodies should be evaluated further in vitro using authentic EBOV and in vivo in animal models of EVD before their therapeutic/prophylactic effectiveness is clinically evaluated.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
