Enhanced hexose-6-phosphate dehydrogenase expression in adipose tissue may contribute to diet-induced visceral adiposity
- PMID: 29568102
- PMCID: PMC6105561
- DOI: 10.1038/s41366-018-0041-1
Enhanced hexose-6-phosphate dehydrogenase expression in adipose tissue may contribute to diet-induced visceral adiposity
Abstract
Background: Visceral fat accumulation increases the risk of developing type 2 diabetes and metabolic syndrome, and is associated with excessive glucocorticoids (GCs). Fat depot-specific GC action is tightly controlled by 11ß-hydroxysteroid dehydrogenase (11ß-HSD1) coupled with the enzyme hexose-6-phosphate dehydrogenase (H6PDH). Mice with inactivation or activation of H6PDH genes show altered adipose 11ß-HSD1 activity and lipid storage. We hypothesized that adipose tissue H6PDH activation is a leading cause for the visceral obesity and insulin resistance. Here, we explored the role and possible mechanism of enhancing adipose H6PDH in the development of visceral adiposity in vivo.
Methods: We investigated the potential contribution of adipose H6PDH activation to the accumulation of visceral fat by characterization of visceral fat obese gene expression profiles, fat distribution, adipocyte metabolic molecules, and abdominal fat-specific GC signaling mechanisms underlying the diet-induced visceral obesity and insulin resistance in H6PDH transgenic mice fed a standard of high-fat diet (HFD).
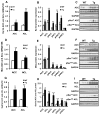
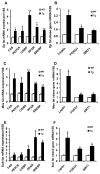
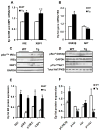
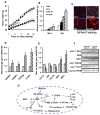
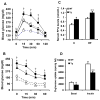
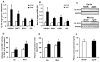
Results: Transgenic H6PDH mice display increased abdominal fat accumulation, which is paralleled by elevated lipid synthesis associated with induction of lipogenic transcriptor C/EBPα and PPARγ mRNA levels within adipose tissue. Transgenic H6PDH mice fed a high-fat diet (HFD) gained more abdominal visceral fat mass coupled with activation of GSK3β and induction of XBP1/IRE1α, but reduced pThr308 Akt/PKB content and browning gene CD137 and GLUT4 mRNA levels within the visceral adipose tissue than WT controls. HFD-fed H6PDH transgenic mice also had impaired insulin sensitivity and exhibited elevated levels of intra-adipose GCs with induction of adipose 11ß-HSD1.
Conclusion: These data provide the first in vivo mechanistic evidence for the adverse metabolic effects of adipose H6PDH activation on visceral fat distribution, fat metabolism, and adipocyte function through enhancing 11ß-HSD1-driven intra-adipose GC action.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
Lack of adipose-specific hexose-6-phosphate dehydrogenase causes inactivation of adipose glucocorticoids and improves metabolic phenotype in mice.Clin Sci (Lond). 2019 Nov 15;133(21):2189-2202. doi: 10.1042/CS20190679. Clin Sci (Lond). 2019. PMID: 31696216 Free PMC article.
-
Increased glycogen synthase kinase-3β and hexose-6-phosphate dehydrogenase expression in adipose tissue may contribute to glucocorticoid-induced mouse visceral adiposity.Int J Obes (Lond). 2016 Aug;40(8):1233-41. doi: 10.1038/ijo.2016.57. Epub 2016 Apr 22. Int J Obes (Lond). 2016. PMID: 27102048 Free PMC article.
-
Transgenic overexpression of hexose-6-phosphate dehydrogenase in adipose tissue causes local glucocorticoid amplification and lipolysis in male mice.Am J Physiol Endocrinol Metab. 2014 Mar 1;306(5):E543-51. doi: 10.1152/ajpendo.00491.2013. Epub 2013 Dec 31. Am J Physiol Endocrinol Metab. 2014. PMID: 24381005 Free PMC article.
-
Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity.Biochim Biophys Acta. 2014 Mar;1842(3):473-81. doi: 10.1016/j.bbadis.2013.05.029. Epub 2013 Jun 2. Biochim Biophys Acta. 2014. PMID: 23735216 Free PMC article. Review.
-
Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)--a promising drug target for the treatment of metabolic syndrome.Curr Drug Targets Immune Endocr Metabol Disord. 2003 Dec;3(4):255-62. doi: 10.2174/1568008033340135. Curr Drug Targets Immune Endocr Metabol Disord. 2003. PMID: 14683456 Review.
Cited by
-
Decreased 11β-Hydroxysteroid Dehydrogenase Type 2 Expression in the Kidney May Contribute to Nicotine/Smoking-Induced Blood Pressure Elevation in Mice.Hypertension. 2021 Jun;77(6):1940-1952. doi: 10.1161/HYPERTENSIONAHA.120.16458. Epub 2021 Apr 5. Hypertension. 2021. PMID: 33813843 Free PMC article.
-
Glycyrrhizic Acid and Its Derivatives: Promising Candidates for the Management of Type 2 Diabetes Mellitus and Its Complications.Int J Mol Sci. 2022 Sep 20;23(19):10988. doi: 10.3390/ijms231910988. Int J Mol Sci. 2022. PMID: 36232291 Free PMC article. Review.
-
Lack of adipose-specific hexose-6-phosphate dehydrogenase causes inactivation of adipose glucocorticoids and improves metabolic phenotype in mice.Clin Sci (Lond). 2019 Nov 15;133(21):2189-2202. doi: 10.1042/CS20190679. Clin Sci (Lond). 2019. PMID: 31696216 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) influences adipocytes injuries through triggering XBP1 and activating mitochondria-mediated apoptosis.Adipocyte. 2021 Dec;10(1):28-37. doi: 10.1080/21623945.2020.1856527. Adipocyte. 2021. PMID: 33393852 Free PMC article.
References
-
- Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. - PubMed
-
- Himabukuro M. Cardiac adiposity and global cardiometabolic risk: new concept and clinical implication. Circ J. 2009;73:27–34. - PubMed
-
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. - PubMed
-
- Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349:1210–1213. - PubMed
-
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

