The cellular metabolic landscape in the tumor milieu regulates the activity of myeloid infiltrates
- PMID: 29568118
- PMCID: PMC6068094
- DOI: 10.1038/s41423-018-0001-7
The cellular metabolic landscape in the tumor milieu regulates the activity of myeloid infiltrates
Abstract
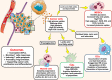
Malignant cells upregulate distinct energy metabolism programs that support their proliferation, migration, and adaptation to the stressful tumor microenvironment (TME). Additionally, this exaggerated metabolic activity allows cancer cells to hijack essential nutrients and outcompete neighboring infiltrating immune cells, thereby impairing antitumor immunity. During recent years, there has been great interest in the field to understand the tumor-induced energy metabolism signals that regulate the function of immune cells in individuals with cancer. Accordingly, it is now well accepted that uncovering the mechanisms that instruct the metabolic behavior of cancer cells and tumor-associated immune cells is an indispensable strategy for the development of new approaches to overcome immune suppression in tumors. Thus, in this minireview, we briefly discuss the interaction between particular metabolic signaling pathways and immunosuppressive activity in different subsets of myeloid cells within the TME. Additionally, we illustrate potential central mechanisms controlling the metabolic reprogramming of myeloid cells in response to tumor-derived factors.
Keywords: Tumor microenvironment; immune suppression; metabolic reprogramming; myeloid cells.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Metabolic Control of Myeloid Cells in the Tumor Microenvironment.Cells. 2021 Oct 30;10(11):2960. doi: 10.3390/cells10112960. Cells. 2021. PMID: 34831183 Free PMC article. Review.
-
Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer.J Immunother Cancer. 2017 Jan 17;5:5. doi: 10.1186/s40425-016-0203-4. eCollection 2017. J Immunother Cancer. 2017. PMID: 28105371 Free PMC article. Review.
-
Metabolic Regulation of Myeloid-Derived Suppressor Cell Function in Cancer.Cells. 2020 Apr 18;9(4):1011. doi: 10.3390/cells9041011. Cells. 2020. PMID: 32325683 Free PMC article. Review.
-
Immunosuppressive Immature Myeloid Cell Generation Is Controlled by Glutamine Metabolism in Human Cancer.Cancer Immunol Res. 2019 Oct;7(10):1605-1618. doi: 10.1158/2326-6066.CIR-18-0902. Epub 2019 Aug 6. Cancer Immunol Res. 2019. PMID: 31387898
-
Myeloid Cell-Derived Oxidized Lipids and Regulation of the Tumor Microenvironment.Cancer Res. 2022 Jan 15;82(2):187-194. doi: 10.1158/0008-5472.CAN-21-3054. Epub 2021 Nov 11. Cancer Res. 2022. PMID: 34764204 Free PMC article. Review.
Cited by
-
Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery.J Exp Clin Cancer Res. 2024 Jan 20;43(1):23. doi: 10.1186/s13046-024-02949-5. J Exp Clin Cancer Res. 2024. PMID: 38245798 Free PMC article. Review.
-
Intracellular Lipid Accumulation Drives the Differentiation of Decidual Polymorphonuclear Myeloid-Derived Suppressor Cells via Arachidonic Acid Metabolism.Front Immunol. 2022 May 18;13:868669. doi: 10.3389/fimmu.2022.868669. eCollection 2022. Front Immunol. 2022. PMID: 35664000 Free PMC article.
-
Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab.Signal Transduct Target Ther. 2021 Jun 23;6(1):236. doi: 10.1038/s41392-021-00629-w. Signal Transduct Target Ther. 2021. PMID: 34158475 Free PMC article.
-
The Unfolded Protein Response Mediator PERK Governs Myeloid Cell-Driven Immunosuppression in Tumors through Inhibition of STING Signaling.Immunity. 2020 Apr 14;52(4):668-682.e7. doi: 10.1016/j.immuni.2020.03.004. Immunity. 2020. PMID: 32294407 Free PMC article.
-
Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy.Front Immunol. 2020 Jun 23;11:1295. doi: 10.3389/fimmu.2020.01295. eCollection 2020. Front Immunol. 2020. PMID: 32714324 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

