Identification of Antidiabetic Compounds from Polyphenolic-rich Fractions of Bulbine abyssinica A. Rich Leaves
- PMID: 29568191
- PMCID: PMC5855377
- DOI: 10.4103/pr.pr_55_17
Identification of Antidiabetic Compounds from Polyphenolic-rich Fractions of Bulbine abyssinica A. Rich Leaves
Abstract
Background: Bulbine abyssinica has been reported to possess a variety of pharmacological activities traditionally. Previous work suggested its antidiabetic properties, but information on the antidiabetic compounds is still lacking.
Objective: The present research exertion was aimed to isolate and identify biologically active polyphenols from B. abyssinica leaves and to evaluate their efficacy on carbohydrate digesting enzymes.
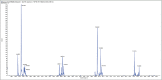
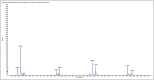
Materials and methods: Fractionation of the polyphenolic contents from the methanolic extract of B. abyssinica leaves was executed by the silica gel column chromatography to yield different fractions. The antioxidant activities of these fractions were carried out against 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl radicals, and ferric ion-reducing antioxidant power (FRAP). In vitro antidiabetic experimentation was performed by evaluating the α-amylase and α-glucosidase inhibitory capacity. The isolated polyphenols were then identified using liquid chromatography and mass spectroscopy (LC/MS).
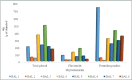
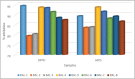
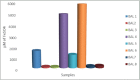
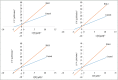
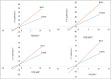
Results: Out of the eight polyphenolic fractions (BAL 1-8), BAL-4 has the highest inhibitory activity against ABTS radicals whereas BAL-6 showed potent ferric ion-reducing capacity. BAL-5 was the most effective fraction with antidiabetic activity with IC50of 140.0 and 68.58 ± 3.2 μg/ml for α-amylase and α-glucosidase inhibitory activities, respectively. All the fractions competitively inhibited α-amylase, BAL-5 and BAL-6 also inhibited α-glucosidase competitively, while BAL-4 and BAL-1 exhibited noncompetitive and near competitive inhibitions against α-glucosidase, respectively. The LC/MS analysis revealed the presence of carvone in all the fractions.
Conclusions: The present study demonstrates the antioxidant and antidiabetic activities of the isolated polyphenols from B. abyssinica.
Summary: Polyphenols were successfully isolated and identified from Bulbine abyssinica leavesThe isolated polyphenols are biologically active with high antioxidant as well as inhibitor of carbohydrate-digesting enzymesB. abyssinica can be a good source of amylase and glucosidase inhibitorsB. abyssinica can be used as complementary or alternative therapeutic agents especially for the treatment of diabetesCarvone, quercetin, and psoralen could be the compounds responsible for the α-amylase and α-glucosidase inhibitory activities. Abbreviations Used: ABTS: 2,2'-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid), DPPH: 2,2-diphenyl-1-picrylhydrazyl, FRAP: Ferric ion-reducing antioxidant power, LC/MS: Liquid chromatography and mass spectroscopy, AGEs: Advanced glycation end products, TLC: Thin-layer chromatography, MeOH: Methanol, PNP-G: ρ-Nitrophenyl-α-D-Glucoside, R2: Coefficient of determination, mgQE: Milligram quercetin equivalent, mgTAE: Milligram tannic acid equivalent, mgCE: Milligram catechin equivalent, g: Gram.
Keywords: Antidiabetics; Bulbine abyssinica; antioxidants; liquid chromatography and mass spectroscopy; medicinal plants; polyphenolics.
Conflict of interest statement
There are no conflicts of interest.
Figures


Similar articles
-
Phytochemical Compositions and In vitro Assessments of Antioxidant and Antidiabetic Potentials of Fractions from Ehretia cymosa Thonn.Pharmacogn Mag. 2017 Oct;13(Suppl 3):S470-S480. doi: 10.4103/pm.pm_118_17. Epub 2017 Oct 11. Pharmacogn Mag. 2017. PMID: 29142401 Free PMC article.
-
Antioxidant and Anti-Proliferative Properties of Hagenia abyssinica Roots and Their Potentially Active Components.Antioxidants (Basel). 2020 Feb 6;9(2):143. doi: 10.3390/antiox9020143. Antioxidants (Basel). 2020. PMID: 32041310 Free PMC article.
-
In Vitro α-Amylase and α-Glucosidase Inhibitory and Antioxidant Activities of the Crude Extract and Solvent Fractions of Hagenia abyssinica Leaves.Biomed Res Int. 2021 Apr 19;2021:6652777. doi: 10.1155/2021/6652777. eCollection 2021. Biomed Res Int. 2021. PMID: 33987444 Free PMC article.
-
Comparative Analysis of Lespedeza Species: Traditional Uses and Biological Activity of the Fabaceae Family.Molecules. 2025 Apr 30;30(9):2013. doi: 10.3390/molecules30092013. Molecules. 2025. PMID: 40363818 Free PMC article. Review.
-
Pharmacological evaluation of medicinal plants with antidiabetic activities in Ethiopia: A review.Metabol Open. 2022 Mar 10;13:100174. doi: 10.1016/j.metop.2022.100174. eCollection 2022 Mar. Metabol Open. 2022. PMID: 35296054 Free PMC article. Review.
Cited by
-
Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology.Molecules. 2018 Oct 25;23(11):2759. doi: 10.3390/molecules23112759. Molecules. 2018. PMID: 30366359 Free PMC article. Review.
-
In Vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract.Plants (Basel). 2022 Dec 26;12(1):112. doi: 10.3390/plants12010112. Plants (Basel). 2022. PMID: 36616242 Free PMC article.
-
Repression of Acetaminophen-Induced Hepatotoxicity in HepG2 Cells by Polyphenolic Compounds from Lauridia tetragona (L.f.) R.H. Archer.Molecules. 2019 Jun 4;24(11):2118. doi: 10.3390/molecules24112118. Molecules. 2019. PMID: 31167480 Free PMC article.
-
The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders.Molecules. 2020 Jun 9;25(11):2669. doi: 10.3390/molecules25112669. Molecules. 2020. PMID: 32526850 Free PMC article. Review.
-
Synergistic anti-diabetic effect of phloroglucinol and total procyanidin dimer isolated from Vitisvinifera methanolic seed extract potentiates via suppressing oxidative stress: in-vitro evaluation studies.3 Biotech. 2024 Mar;14(3):76. doi: 10.1007/s13205-024-03929-4. Epub 2024 Feb 14. 3 Biotech. 2024. PMID: 38371900 Free PMC article.
References
-
- Mazid M, Khan T, Mohammad F. Role of secondary metabolites in defense mechanisms of plants. Biol Med. 2011;3:232–9.
-
- Wuyts N, De Waele D, Swennen R. Extraction and partial characterization of polyphenol oxidase from banana (Musa acuminata grande naine) roots. Plant Physiol Biochem. 2006;44:308–14. - PubMed
-
- Genwali GR, Acharya PP, Rajbhandari M. Isolation of gallic acid and estimation of total phenolic content in some medicinal plants and their antioxidant activity. Nepal J Sci Technol. 2013;14:95–102.
-
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. - PubMed
-
- Odeyemi S, Afolayan A, Bradley G. In vitro anti-inflammatory and free radical scavenging activities of crude saponins extracted from Albuca bracteata Jacq. Bulb. Afr J Tradit Complement Altern Med. 2015;12:34–40.
LinkOut - more resources
Full Text Sources
Miscellaneous
