Trying to See the Forest through the Trees: Deciphering the Nature of Memory Immunity to Mycobacterium tuberculosis
- PMID: 29568298
- PMCID: PMC5852080
- DOI: 10.3389/fimmu.2018.00461
Trying to See the Forest through the Trees: Deciphering the Nature of Memory Immunity to Mycobacterium tuberculosis
Abstract
The purpose of vaccination against tuberculosis and other diseases is to establish a heightened state of acquired specific resistance in which the memory immune response is capable of mediating an accelerated and magnified expression of protection to the pathogen when this is encountered at a later time. In the earliest studies in mice infected with Mycobacterium tuberculosis, memory immunity and the cells that express this were definable both in terms of kinetics of emergence, and soon thereafter by the levels of expression of markers including CD44, CD62L, and the chemokine receptor CCR7, allowing the identification of effector memory and central memory T cell subsets. Despite these initial advances in knowledge, more recent information has not revealed more clarity, but instead, has created a morass of complications-complications that, if not resolved, could harm correct vaccine design. Here, we discuss two central issues. The first is that we have always assumed that memory is induced in the same way, and consists of the same T cells, regardless of whether that immunity is generated by BCG vaccination, or by exposure to M. tuberculosis followed by effective chemotherapy. This assumption is almost certainly incorrect. Second, a myriad of additional memory subsets have now been described, such as resident, stem cell-like, tissue specific, among others, but as yet we know nothing about the relative importance of each, or whether if a new vaccine needs to induce all of these, or just some, to be fully effective.
Keywords: BCG; Mycobacterium tuberculosis; memory T cell subsets; memory immunity; vaccination.
Figures

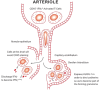
References
-
- Kirman JR, Henao Tamayo MI, Agger EM. The memory immune response to tuberculosis. 2nd ed In: Jacobs WR, Jr, McShane H, Mizrahi V, Orme IM, editors. Tuberculosis and the Tubercle Bacillus. Washington, DC: ASM Press; (2017). p. 95–116.
-
- Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol (1987) 138(1):293–8. - PubMed
-
- Orme IM. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol (1988) 140(10):3589–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

