Synthetic pathways and processes for effective production of 5-hydroxytryptophan and serotonin from glucose in Escherichia coli
- PMID: 29568327
- PMCID: PMC5856393
- DOI: 10.1186/s13036-018-0094-7
Synthetic pathways and processes for effective production of 5-hydroxytryptophan and serotonin from glucose in Escherichia coli
Abstract
Background: Tryptophan derivatives such as 5-hydroxytryptophan (5HTP) and serotonin are valuable molecules with pharmaceutical interest. 5HTP is presently mainly obtained by extraction from the plant Griffonia simplicifolia and serotonin is produced by chemical synthesis. A simple biotechnological method for the production of these compounds is desired.
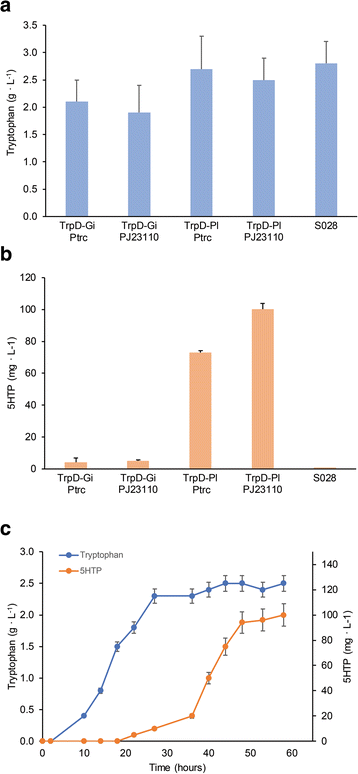
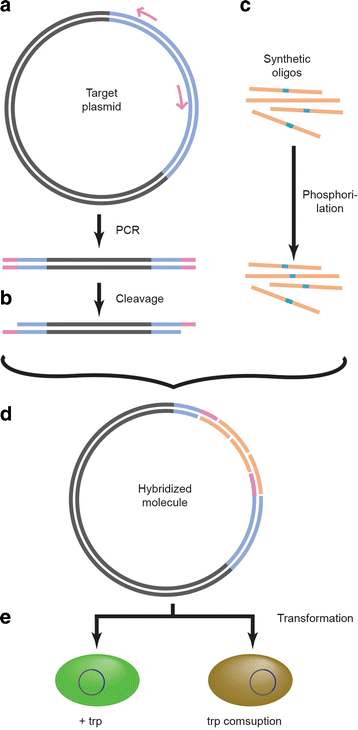
Results: In a first attempt to synthesize serotonin from glucose, we used a single engineered Escherichia coli strain and observed a low production of maximal 0.8 ± 0.2 mg/L of serotonin, probably due to the undesired site-reaction of direct decarboxylation of tryptophan and the consequent decrease of the precursor 5HTP. To circumvent this problem, we have constructed a stepwise system in which the 5HTP production and the serotonin conversion are separated. 962 ± 58 mg/L of 5HTP was produced in the first step using a recombinant strain with a semi-rationally engineered aromatic amino acid hydroxylase, the highest concentration reported so far. In a subsequent step of 5HTP bioconversion using a recombinant strain harboring a tryptophan decarboxylase, 154.3 ± 14.3 mg/L of serotonin was produced.
Conclusions: We present results of a two-stage fermentation process for the production of 5HTP and serotonin. The first strain is a highly efficient 5HTP producer, and after fermentation the supernatant is separated and used for the production of serotonin. This is the first report for the microbial production of serotonin from glucose.
Keywords: 5-hydroxytryptophan; Aromatic amino acid hydroxylase; Protein engineering; Serotonin; Synthetic pathway.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Metabolic engineering of Escherichia coli for efficient production of L-5-hydroxytryptophan from glucose.Microb Cell Fact. 2022 Sep 24;21(1):198. doi: 10.1186/s12934-022-01920-3. Microb Cell Fact. 2022. PMID: 36153615 Free PMC article.
-
Protein and pathway engineering for the biosynthesis of 5-hydroxytryptophan in Escherichia coli.Eng Life Sci. 2017 Jun 21;17(8):892-899. doi: 10.1002/elsc.201700064. eCollection 2017 Aug. Eng Life Sci. 2017. PMID: 32624837 Free PMC article.
-
[Cerebral serotonin and the hypothalamo-hypophyseal-adrenal system of the rat during aging].Rev Esp Fisiol. 1991 Dec;47(4):187-91. Rev Esp Fisiol. 1991. PMID: 1667440 Spanish.
-
Advances in the microbial synthesis of the neurotransmitter serotonin.Appl Microbiol Biotechnol. 2023 Aug;107(15):4717-4725. doi: 10.1007/s00253-023-12584-3. Epub 2023 Jun 16. Appl Microbiol Biotechnol. 2023. PMID: 37326681 Review.
-
5-Hydroxytryptophan: a clinically-effective serotonin precursor.Altern Med Rev. 1998 Aug;3(4):271-80. Altern Med Rev. 1998. PMID: 9727088 Review.
Cited by
-
The role of tryptophan derivatives as anti-kinetoplastid agents.Heliyon. 2023 Dec 15;10(1):e23895. doi: 10.1016/j.heliyon.2023.e23895. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187297 Free PMC article. Review.
-
Construction of cell factory capable of efficiently converting L-tryptophan into 5-hydroxytryptamine.Microb Cell Fact. 2022 Mar 24;21(1):47. doi: 10.1186/s12934-022-01745-0. Microb Cell Fact. 2022. PMID: 35331215 Free PMC article.
-
Recent Advances in Metabolically Engineered Microorganisms for the Production of Aromatic Chemicals Derived From Aromatic Amino Acids.Front Bioeng Biotechnol. 2020 May 5;8:407. doi: 10.3389/fbioe.2020.00407. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32432104 Free PMC article. Review.
-
Advances in the Microbial Synthesis of 5-Hydroxytryptophan.Front Bioeng Biotechnol. 2021 Feb 3;9:624503. doi: 10.3389/fbioe.2021.624503. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33634088 Free PMC article. Review.
-
Metabolic engineering of microorganisms for production of aromatic compounds.Microb Cell Fact. 2019 Feb 26;18(1):41. doi: 10.1186/s12934-019-1090-4. Microb Cell Fact. 2019. PMID: 30808357 Free PMC article. Review.
References
-
- Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998;3:271–280. - PubMed
-
- Gong F, Li C, Xu Y. Novel simple synthesis method of L-5-hydroxytryptophan. China; 2013;CN Patent App. CN 201310592943.
-
- Frangatos G, Chubb FL. A new synthesis of 5-hydroxytryptophan. Can J Chem. 1959;37:1374–1376. doi: 10.1139/v59-200. - DOI
-
- Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006:325–38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

