miR-128 inhibits telomerase activity by targeting TERT mRNA
- PMID: 29568354
- PMCID: PMC5862575
- DOI: 10.18632/oncotarget.24284
miR-128 inhibits telomerase activity by targeting TERT mRNA
Abstract
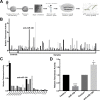
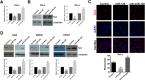
Telomerase is a unique cellular reverse transcriptase (RT) essential for maintaining telomere stability and required for the unlimited proliferation of cancer cells. The limiting determinant of telomerase activity is the catalytic component TERT, and TERT expression is closely correlated with telomerase activity and cancer initiation and disease progression. For this reason the regulation of TERT levels in the cell is of great importance. microRNAs (miRs) function as an additional regulatory level in cells, crucial for defining expression boundaries, proper cell fate decisions, cell cycle control, genome integrity, cell death and metastasis. We performed an anti-miR library screen to identity novel miRs, which participate in the control of telomerase. We identified the tumor suppressor miR (miR-128) as a novel endogenous telomerase inhibitor and determined that miR-128 significantly reduces the mRNA and protein levels of Tert in a panel of cancer cell lines. We further evaluated the mechanism by which miR-128 regulates TERT and demonstrated that miR-128 interacts directly with the coding sequence of TERT mRNA in both HeLa cells and teratoma cells. Interestingly, the functional miR-128 binding site in TERT mRNA, is conserved between TERT and the other cellular reverse transcriptase encoded by Long Interspersed Elements-1 (LINE-1 or L1), which can also contribute to the oncogenic phenotype of cancer. This finding supports the novel idea that miRs may function in parallel pathways to inhibit tumorigenesis, by regulating a group of enzymes (such as RT) by targeting conserved binding sites in the coding region of both enzymes.
Keywords: TERT; cancer; miR; miR-128; telomerase.
Conflict of interest statement
CONFLICTS OF INTEREST All co-authors implicated in this research approved of this article to be published. No authors have declared any conflicts of interest.
Figures

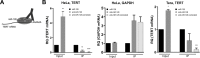
Similar articles
-
Feedback regulation of telomerase reverse transcriptase: new insight into the evolving field of telomerase in cancer.Cell Signal. 2013 Dec;25(12):2462-8. doi: 10.1016/j.cellsig.2013.08.009. Epub 2013 Aug 29. Cell Signal. 2013. PMID: 23993966 Review.
-
Modulation of telomerase expression and function by miRNAs: Anti-cancer potential.Life Sci. 2020 Oct 15;259:118387. doi: 10.1016/j.lfs.2020.118387. Epub 2020 Sep 3. Life Sci. 2020. PMID: 32890603 Review.
-
Quantitative and spatial measurements of telomerase reverse transcriptase expression within normal and malignant human breast tissues.Mol Cancer Res. 2005 Sep;3(9):503-9. doi: 10.1158/1541-7786.MCR-05-0031. Mol Cancer Res. 2005. PMID: 16179497
-
CTCF mediates the TERT enhancer-promoter interactions in lung cancer cells: identification of a novel enhancer region involved in the regulation of TERT gene.Int J Cancer. 2014 May 15;134(10):2305-13. doi: 10.1002/ijc.28570. Epub 2013 Nov 14. Int J Cancer. 2014. PMID: 24174344
-
Pan-cancer analysis identifies telomerase-associated signatures and cancer subtypes.Mol Cancer. 2019 Jun 10;18(1):106. doi: 10.1186/s12943-019-1035-x. Mol Cancer. 2019. PMID: 31179925 Free PMC article.
Cited by
-
MicroRNA biomarkers as next-generation diagnostic tools for neurodegenerative diseases: a comprehensive review.Front Mol Neurosci. 2024 May 31;17:1386735. doi: 10.3389/fnmol.2024.1386735. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38883980 Free PMC article. Review.
-
Mechanism of Human Telomerase Reverse Transcriptase (hTERT) Regulation and Clinical Impacts in Leukemia.Genes (Basel). 2021 Jul 30;12(8):1188. doi: 10.3390/genes12081188. Genes (Basel). 2021. PMID: 34440361 Free PMC article. Review.
-
Telomeres and telomerase in oncogenesis.Oncol Lett. 2020 Aug;20(2):1015-1027. doi: 10.3892/ol.2020.11659. Epub 2020 May 21. Oncol Lett. 2020. PMID: 32724340 Free PMC article. Review.
-
Beginning at the ends: telomere and telomere-based cancer therapeutics.Mol Genet Genomics. 2024 Dec 6;300(1):1. doi: 10.1007/s00438-024-02206-6. Mol Genet Genomics. 2024. PMID: 39638969 Review.
-
The role of miR-128 in cancer development, prevention, drug resistance, and immunotherapy.Front Oncol. 2023 Jan 19;12:1067974. doi: 10.3389/fonc.2022.1067974. eCollection 2022. Front Oncol. 2023. PMID: 36793341 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. - PubMed
-
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. - PubMed
-
- Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

