Methotrexate sensitizes drug-resistant metastatic melanoma cells to BRAF V600E inhibitors dabrafenib and encorafenib
- PMID: 29568360
- PMCID: PMC5862581
- DOI: 10.18632/oncotarget.24341
Methotrexate sensitizes drug-resistant metastatic melanoma cells to BRAF V600E inhibitors dabrafenib and encorafenib
Abstract
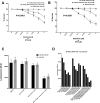
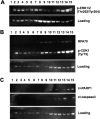
Acquired resistance of metastatic melanoma (MM) tumors to BRAF V600E inhibitors (BRAFi's) is commonplace in the clinic. Habitual relapse of patients contributes to <20% 5-year survival rates in MM. We previously identified serine synthesis as a critical detrminant of late-stage cancer cell resistance to BRAFi's. Pre-treatment with DNA damaging agent gemcitabine (a nucleoside analog) re-sensitized drug-resistant cancer cells to BRAFi's dabrafenib and vemurafenib. Importantly, the combination treatments were effective against BRAF wild type cancer cells potentially expanding the clinical reach of BRAFi's. In this study, we identify the antifolate methotrexate (MTX) as a sensitizer of acquired- and intrinsically-resistant MM cells to BRAFi's dabrafenib and encorafenib. We identify a novel, positive correlation between dabrafenib treatments and repair delay of MTX induced single-strand DNA (ssDNA) breaks. Cells arrest in G1 phase following simultaneous MTX + dabrafenib treatments and eventually die via apoptosis. Importantly, we identify RAS codon 12 activating mutations as prognostic markers for MTX + BRAFi treatment efficacy. We describe a method of killing drug-resistant MM cells that if translated has the potential to improve MM patient survival.
Keywords: dabrafenib; encorafenib; metastatic melanoma; methotrexate; pancreatic cancer.
Conflict of interest statement
CONFLICTS OF INTEREST T.J. Yen is a consultant/advisory board member for Evol Science. No potential conflicts of interest were disclosed by the other authors.
Figures

References
-
- Ross KC, Andrews AJ, Marion CD, Yen TJ, Bhattacharjee V. Identification of the serine biosynthesis pathway as a critical component of BRAF inhibitor resistance of melanoma, pancreatic, and non-small cell lung cancer cells. Mol Cancer Ther. 2017;16:1596–1609. https://doi.org/10.1158/1535-7163.MCT-16-0798. - DOI - PMC - PubMed
-
- Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–9. https://doi.org/10.1016/j.bcp.2011.05.015. - DOI - PMC - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. https://doi.org/10.1038/nature00766. - DOI - PubMed
-
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, Roth JA, Albelda SM, Davies H, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. - PubMed
-
- Spagnolo F, Ghiorzo P, Queirolo P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget. 2014;5:10206–21. https://doi.org/10.18632/oncotarget.2602. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

