Amiodarone promotes cancer cell death through elevated truncated SRSF3 and downregulation of miR-224
- PMID: 29568365
- PMCID: PMC5862586
- DOI: 10.18632/oncotarget.24385
Amiodarone promotes cancer cell death through elevated truncated SRSF3 and downregulation of miR-224
Abstract
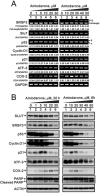
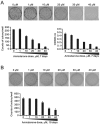
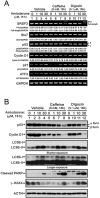
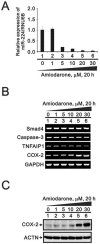
Amiodarone is a widely used class III antiarrhythmic agent which prolongs the action potential and refractory period by blockage of several types of myocardial potassium channels. Emerging evidence suggests that amiodarone sensitize tumor cells in response to chemotherapy. Nevertheless, little is known about the underlying molecular mechanism. To gain further insight, we demonstrated that amiodarone accumulated the population of a premature termination codon-containing isoform of serine and arginine rich splicing factor 3 (SRSF3-PTC) without increasing alternative spliced p53 beta isoform. Amiodarone enhanced reactive oxygen species production and increased cell apoptosis, whereas reduced DNA damage. Moreover, amiodarone suppressed miR-224 and increased its target COX-2 expression. Taken together, our results suggested amiodarone caused cancer cell death might be through increased SRSF3-PTC and miR-224 reduction in a p53-independent manner.
Keywords: amiodarone; autophagy; caffeine; digoxin; miR-224.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interests related to this study.
Figures







References
-
- Ellison KE, Stevenson WG, Sweeney MO, Epstein LM, Maisel WH. Management of arrhythmias in heart failure. Congest Heart Fail. 2003;9:91–99. - PubMed
-
- Singh BN. Current antiarrhythmic drugs: an overview of mechanisms of action and potential clinical utility. J Cardiovasc Electrophysiol. 1999;10:283–301. - PubMed
-
- Singh BN. Amiodarone as paradigm for developing new drugs for atrial fibrillation. J Cardiovasc Pharmacol. 2008;52:300–305. - PubMed
-
- Kathofer S, Thomas D, Karle CA. The novel antiarrhythmic drug dronedarone: comparison with amiodarone. Cardiovasc Drug Rev. 2005;23:217–230. - PubMed
-
- Karavelioglu Y, Karapinar H, Yuksel M, Memic K, Sarak T, Kurt R, Yilmaz A. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. 2015;21:5–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

