Persistent DNA strand breaks induce a CAF-like phenotype in normal fibroblasts
- PMID: 29568385
- PMCID: PMC5862606
- DOI: 10.18632/oncotarget.24446
Persistent DNA strand breaks induce a CAF-like phenotype in normal fibroblasts
Abstract
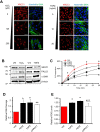
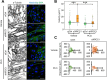
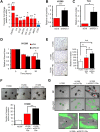
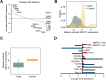
Cancer-associated fibroblasts (CAFs) are an emerging target for cancer therapy as they promote tumour growth and metastatic potential. However, CAF targeting is complicated by the lack of knowledge-based strategies aiming to selectively eliminate these cells. There is a growing body of evidence suggesting that a pro-inflammatory microenvironment (e.g. ROS and cytokines) promotes CAF formation during tumorigenesis, although the exact mechanisms involved remain unclear. In this study, we reveal that a prolonged pro-inflammatory stimulation causes a de facto deficiency in base excision repair, generating unrepaired DNA strand breaks and thereby triggering an ATF4-dependent reprogramming of normal fibroblasts into CAF-like cells. Based on the phenotype of in vitro-generated CAFs, we demonstrate that midostaurin, a clinically relevant compound, selectively eliminates CAF-like cells deficient in base excision repair and prevents their stimulatory role in cancer cell growth and migration.
Keywords: base excision repair; cancer-associated fibroblasts; midostaurin; tumour microenvironment; tumour stroma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. - PubMed
-
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. - PubMed
-
- Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6. - PubMed
-
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. - PubMed
-
- Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

