Chemopreventive effects of angiotensin II receptor type 2 agonist on prostate carcinogenesis by the down-regulation of the androgen receptor
- PMID: 29568400
- PMCID: PMC5862621
- DOI: 10.18632/oncotarget.24492
Chemopreventive effects of angiotensin II receptor type 2 agonist on prostate carcinogenesis by the down-regulation of the androgen receptor
Abstract
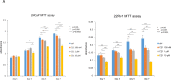
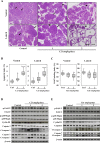
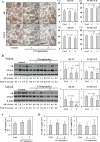
We recently reported that angiotensin II receptor blockers (ARBs) have chemopreventive and chemotherapeutic potential against prostate cancer via the reduction of androgen receptor (AR) expression. In this study, we investigated the effects of the angiotensin II receptor type 2 (AT2R) agonist Compound 21 (C21), which is expected to play similar roles to an ARB, on prostate carcinogenesis using the transgenic rat for adenocarcinoma of prostate (TRAP) model previously established in our laboratory. In vitro analyses of the cell growth, Western blotting and reporter gene assays were performed using LNCaP cells. TRAP rats at 6 weeks of age were randomly divided into 3 groups of 12 animals each and treated with C21 at 1 or 2 mg/kg/day in drinking water for 12 weeks. C21 reduced the proliferation activity of prostate cancer cells and down-regulated the PSA promoter activity and the AR protein expression. We discovered that C21 inhibited the progression of prostate carcinogenesis in TRAP rats and decreased the incidence of adenocarcinoma in the lateral prostate. A significant increase in the apoptotic index with activation of caspase 3 and 7 were observed by immunohistochemistry and Western blotting analyses. C21 also down-regulated the expression of AR significantly in TRAP rat prostate. C21 decreased the expression of AR and reduced the proliferation activity effectively in prostate cancer cells and TRAP rat prostate. These findings suggest that AT2R agonist may be a candidate novel chemopreventive agent against human prostate cancer.
Keywords: RAS; angiotensin II receptor type 2; compound 21; prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Therapeutic targeting of angiotensin II receptor type 1 to regulate androgen receptor in prostate cancer.Prostate. 2012 Oct 1;72(14):1559-72. doi: 10.1002/pros.22505. Epub 2012 Mar 16. Prostate. 2012. PMID: 22430461
-
Expression and role of the angiotensin II AT2 receptor in human prostate tissue: in search of a new therapeutic option for prostate cancer.Prostate. 2013 Jul;73(10):1057-68. doi: 10.1002/pros.22653. Epub 2013 Feb 6. Prostate. 2013. PMID: 23389987
-
Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer.Carcinogenesis. 2020 Aug 12;41(8):1145-1157. doi: 10.1093/carcin/bgz193. Carcinogenesis. 2020. PMID: 31805186 Free PMC article.
-
Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis.Prostate. 2015 Feb;75(2):151-60. doi: 10.1002/pros.22900. Epub 2014 Oct 4. Prostate. 2015. PMID: 25284475
-
Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth.Mol Cell Endocrinol. 2002 Nov 29;197(1-2):231-8. doi: 10.1016/s0303-7207(02)00263-0. Mol Cell Endocrinol. 2002. PMID: 12431817 Review.
Cited by
-
The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: foes versus allies.Cancer Cell Int. 2023 Oct 27;23(1):254. doi: 10.1186/s12935-023-03080-9. Cancer Cell Int. 2023. PMID: 37891636 Free PMC article. Review.
-
The AT1/AT2 Receptor Equilibrium Is a Cornerstone of the Regulation of the Renin Angiotensin System beyond the Cardiovascular System.Molecules. 2023 Jul 18;28(14):5481. doi: 10.3390/molecules28145481. Molecules. 2023. PMID: 37513355 Free PMC article. Review.
-
The Effect of Local Renin Angiotensin System in the Common Types of Cancer.Front Endocrinol (Lausanne). 2021 Sep 3;12:736361. doi: 10.3389/fendo.2021.736361. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34539580 Free PMC article. Review.
-
Angiotensin II and Angiotensin Receptors 1 and 2-Multifunctional System in Cells Biology, What Do We Know?Cells. 2021 Feb 12;10(2):381. doi: 10.3390/cells10020381. Cells. 2021. PMID: 33673178 Free PMC article. Review.
-
The Angiotensin AT2 Receptor: From a Binding Site to a Novel Therapeutic Target.Pharmacol Rev. 2022 Oct;74(4):1051-1135. doi: 10.1124/pharmrev.120.000281. Pharmacol Rev. 2022. PMID: 36180112 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Huggins C. Endocrine-induced regression of cancers. Cancer Res. 1967;27:1925–30. - PubMed
-
- Hoshino K, Ishiguro H, Teranishi J, Yoshida S, Umemura S, Kubota Y, Uemura H. Regulation of androgen receptor expression through angiotensin II type 1 receptor in prostate cancer cells. Prostate. 2011;71:964–75. - PubMed
-
- Ishiguro H, Ishiguro Y, Kubota Y, Uemura H. Regulation of prostate cancer cell growth and PSA expression by angiotensin II receptor blocker with peroxisome proliferator-activated receptor gamma ligand like action. Prostate. 2007;67:924–32. - PubMed
-
- Takahashi S, Uemura H, Seeni A, Tang M, Komiya M, Long N, Ishiguro H, Kubota Y, Shirai T. Therapeutic targeting of angiotensin II receptor type 1 to regulate androgen receptor in prostate cancer. Prostate. 2012;72:1559–72. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

